Obstructive sleep apnea
| Obstructive sleep apnea | |
|---|---|
| Other names | Obstructive sleep apnoea |
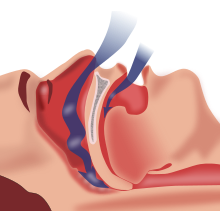 | |
| Obstructive sleep apnea: As soft tissue falls to the back of the throat, it impedes the passage of air (blue arrows) through the trachea. | |
| Specialty | Sleep medicine |
Obstructive sleep apnea (OSA) is the most common sleep-related breathing disorder and is characterized by recurrent episodes of complete or partial obstruction of the upper airway leading to reduced or absent breathing during sleep. These episodes are termed "apneas" with complete or near-complete cessation of breathing, or "hypopneas" when the reduction in breathing is partial. In either case, a fall in blood oxygen saturation, a disruption in sleep, or both, may result. A high frequency of apneas or hypopneas during sleep may interfere with the quality of sleep, which – in combination with disturbances in blood oxygenation – is thought to contribute to negative consequences to health and quality of life.[1] The terms obstructive sleep apnea syndrome (OSAS) or obstructive sleep apnea–hypopnea syndrome (OSAHS) may be used to refer to OSA when it is associated with symptoms during the daytime (e.g. excessive daytime sleepiness, decreased cognitive function).[2][3]
Most individuals with obstructive sleep apnea are unaware of disturbances in breathing while sleeping, even after awakening. A bed partner or family member may observe a person snoring or appear to stop breathing, gasp, or choke while sleeping. People who live or sleep alone are often unaware of the condition. Symptoms may be present for years or even decades without identification, during which time the person may become conditioned to the daytime sleepiness, headaches and fatigue associated with significant levels of sleep disturbance. Obstructive sleep apnea has been associated with neurocognitive morbidity and there is a link between snoring and neurocognitive disorders.[4]
Classification
[edit]In the third edition of the International Classification of Sleep Disorders (ICSD-3), obstructive sleep apnea is classified amongst the sleep-related breathing disorders and is divided in two categories, namely adult OSA and pediatric OSA.[5] Obstructive sleep apnea is differentiated from central sleep apnea (CSA), which is characterized by episodes of reduction or cessation in breathing attributable to decreased effort, rather than upper airway obstruction.[6] The respiratory effort must then be assessed in order to correctly classify the apnea as obstructive given the specificity of the diaphragmatic activity in this condition:[7] the inspiratory effort is continued or increased through the entire episode of absent airflow.[8]
When hypopneas are present alongside apneas, the term obstructive sleep apnea-hypopnea is used and when it is associated with daytime sleepiness and other daytime symptoms, it is called obstructive sleep apnea-hypopnea syndrome.[9] To be categorized as obstructive, the hypopnea must meet one or more of the following symptoms: (1) snoring during the event, (2) increased oronasal flow flattening, or (3) thoraco-abdominal paradoxical respiration during the event.[8] If none of them are present during the event, then it is categorized as central hypopnea.
Signs and symptoms
[edit]Common symptoms of OSA syndrome include unexplained daytime sleepiness, restless sleep, and loud snoring (with periods of silence followed by gasps). Less common symptoms are morning headaches; insomnia; trouble concentrating; mood changes such as irritability, anxiety, and depression; forgetfulness; increased heart rate or blood pressure; decreased sex drive; unexplained weight gain; increased urinary frequency or nocturia; frequent heartburn or gastroesophageal reflux; and heavy night sweats.[10]
Many people experience episodes of OSA transiently, for only a short period of time. This can be the result of an upper respiratory infection that causes nasal congestion, along with swelling of the throat, or tonsillitis that temporarily produces very enlarged tonsils.[11][12] The Epstein-Barr virus, for example, is known to be able to dramatically increase the size of lymphoid tissue during acute infection, and OSA is fairly common in acute cases of severe infectious mononucleosis. Temporary spells of OSA syndrome may also occur in people who are under the influence of a drug (such as alcohol) that may relax their body tone excessively and interfere with normal arousal from sleep mechanisms.[13]
Adults
[edit]The hallmark symptom of OSA syndrome in adults is excessive daytime sleepiness. Typically, an adult or adolescent with severe long-standing OSA will fall asleep for very brief periods in the course of usual daytime activities if given an opportunity to sit or rest. This behavior may be quite dramatic, sometimes occurring during conversations with others at social gatherings.[citation needed]
The hypoxia (absence of oxygen supply) related to OSA may cause changes in the neurons of the hippocampus and the right frontal cortex. Research using neuro-imaging revealed evidence of hippocampal atrophy in people with OSA. They found that OSA can cause problems in mentally manipulating non-verbal information, in executive functions and working memory.[citation needed] This repeated brain hypoxia is also considered to be a cause of Alzheimer's disease.[14]
Diagnosis of obstructive sleep apnea is significantly more common among people in relationships, who are alerted to their condition by being informed by their sleeping partner since individuals with obstructive sleep apnea are often unaware of the condition.[citation needed] There is a stigma associated with loud snoring, and it is not considered a feminine trait. Consequently, females are less likely to be told by their partners that they snore, or to admit it to themselves or doctors.[citation needed] Furthermore, CPAP (Continuous Positive Airway Pressure) machines are also perceived negatively by females, and less likely to be utilized to their full extent in this group.[15]
Children
[edit]Although this so-called "hypersomnolence" (excessive sleepiness) may also occur in children, it is not at all typical of young children with sleep apnea. Toddlers and young children with severe OSA instead ordinarily behave as if "over-tired" or "hyperactive"; and usually appear to have behavioral problems like irritability, and a deficit in attention.[16][17][18]
Adults and children with very severe OSA also differ in typical body habitus. Adults are generally heavy, with particularly short and heavy necks. Young children, on the other hand, are generally not only thin but may have "failure to thrive", where growth is reduced. Poor growth occurs for two reasons: the work of breathing is intense enough that calories are burned at high rates even at rest, and the nose and throat are so obstructed that eating is both tasteless and physically uncomfortable. OSA in children, unlike adults, is often caused by obstructive tonsils and adenoids and may sometimes be cured with tonsillectomy and adenoidectomy.[citation needed]
This problem can also be caused by excessive weight in children. In this case, the symptoms are more like the symptoms adults feel such as restlessness, exhaustion, etc. If adenotonsillar hypertrophy remains the most common cause of OSA in children,[19][20] obesity can also play a role in the pathophysiology of upper airway obstruction during sleep which can lead to OSA, making obese children more likely to develop the condition.[21] The recent epidemic increase of obesity prevalence has thus contributed to changes in the prevalence and in the characteristics of pediatric OSA,[22] the severity of OSA being proportional to the degree of obesity.[23][24]
Obesity leads to the narrowing of upper airway structure due to fatty infiltration and fat deposits in the anterior neck region and cervical structures.[19][22] Alongside with the additional weight loading on the respiratory system, it increases the risk of pharyngeal collapsibility while reducing the intrathoracic volume and diaphragm excursion.[22] Moreover, excessive daytime sleepiness resulting from sleep fragmentation can decrease physical activity and thus lead to weight gain (by sedentary habits or increased food intake to overcome somnolence).[25] The obesity-related obstruction of upper airway structure has led some authors to distinguish between two types of OSA in children:[21][22] type I is associated with marked lymphadenoid hypertrophy without obesity and type II is first associated with obesity and with milder upper airway lymphadenoid hyperplasia. The two types of OSA in children can result in different morbidities and consequences.[21] Studies have shown that weight loss in obese adolescents can reduce sleep apnea and thus the symptoms of OSA.[19][24]
Pathophysiology
[edit]This section needs additional citations for verification. (August 2021) |
The transition from wakefulness to sleep (either REM sleep or NREM sleep) is associated with a reduction in upper-airway muscle tone. During REM sleep, muscle tone of the throat and neck, as well as that of the vast majority of skeletal muscles, are almost completely relaxed. This allows the tongue and soft palate/oropharynx to relax, reducing airway patency and potentially impeding or completely obstructing the flow of air into the lungs during inspiration, resulting in reduced respiratory ventilation. If reductions in ventilation are associated with sufficiently low blood-oxygen levels or with sufficiently high breathing efforts against an obstructed airway, neurological mechanisms may trigger a sudden interruption of sleep, called a neurological arousal. This arousal can cause an individual to gasp for air and awaken.[26] These arousals rarely result in complete awakening but can have a significant negative effect on the restorative quality of sleep. In significant cases of OSA, one consequence is sleep deprivation resulting from the repetitive disruption and recovery of sleep activity. This sleep interruption in Stage 3 (also called slow-wave sleep), and in REM sleep, can interfere with normal growth patterns, healing and immune response, especially in children and young adults.
The fundamental cause of OSA is a blocked upper airway, usually behind the tongue and epiglottis, whereby the otherwise patent airway, in an erect and awake patient, collapses when the patient is lying on his or her back and loses muscle tone upon entering deep sleep.
At the beginning of sleep, a patient is in light sleep and there is no tone loss of throat muscles. Airflow is laminar and soundless. As the upper airway collapse progresses, the obstruction becomes increasingly apparent by the initiation of noisy breathing as air turbulence increases, followed by gradually louder snoring as a Venturi effect forms through the ever-narrowing air passage.
The patient's blood-oxygen saturation gradually falls until cessation of sleep noises, signifying total airway obstruction of airflow, which may last for several minutes.
Eventually, the patient must at least partially awaken from deep sleep into light sleep, automatically regaining general muscle tone. This switch from deep to light to deep sleep can be recorded using ECT monitors.
In light sleep, muscle tone is near normal, the airway spontaneously opens, normal noiseless breathing resumes and blood-oxygen saturation rises. Eventually, the patient reenters deep sleep, upper airway tone is again lost, the patient enters the various levels of noisy breathing and the airway blockage returns.
The cycle of muscle-tone loss and restoration coinciding with periods of deep and light sleep repeats throughout the patient's period of sleep.
The number of apnoea and hypopnoea episodes during any given hour is counted and given a score. If a patient has an average of five or more episodes per hour, mild OSA may be confirmed. An average of 30 or more episodes per hour indicates severe OSA.
Pathophysiological models
[edit]The causes of spontaneous upper-airway blockage are strongly debated by clinical professionals. The areas of thought are divided mostly into three medical groups.
Some pulmonologists and neurologists believe the risk factors to be:
- Advanced age, although OSA occurs in neonates, as with Pierre Robin syndrome, and in all age groups of people.
- Brain injury (temporary or permanent), although this does not account for the 99% of OSA patients who have normal brains and normal lives.
- Decreased muscle tone caused by drugs or alcohol, or caused by neurological disorders. This also would not account for the majority of people with OSA.
- Long-term snoring, which is postulated to potentially induce local nerve lesions in the soft tissues of the pharynx. Snoring may produce traumatic vibrations that may give rise to nerve injuries in the upper airway muscles, further contributing to OSA.[27]
- Increased soft tissue around the airway, often resulting from obesity, though not seen in all patients with OSA.
Some otorhinolaryngologists believe the risk factors to be structural features that give rise to a narrowed airway, such as enlarged tonsils, an enlarged posterior tongue or fat deposits in the neck. Further factors leading to OSA can be impaired nasal breathing, floppy soft palate or a collapsible epiglottis.
Some oral and maxillofacial surgeons believe the risk factors to be a number of primary forms of mandibular hypoplasia, which offers a primary anatomical basis to the development of OSA through glossoptosis. Some maxillofacial surgeons who offer orthognathic surgery for treatment of OSA believe that their treatments offer superior guarantees of cure of OSA.
Risk factors
[edit]Obesity
[edit]It is well known that children, adolescents or adults with OSA are often obese. Obese people show an increase in neck fat tissue which potentiate respiratory obstruction during sleep.[28]
However, people of all ages and sex with normal body mass indices (BMIs) can also demonstrate OSA – and these people do not have significant measures of subdermal or intra neck fat as shown on DEXA scans. It is speculated that they may have increased muscle mass, or alternatively have a tendency to decreased muscle tone potentiating airway collapse during sleep.[citation needed]
However, loss of muscle tone is a key feature of deep sleep anyway, and whilst obesity seems a common association, it is not an invariable state of OSA.
Sleeping supine (on one's back) is also represented as a risk factor for OSA. Clearly, gravity and loss of tongue and throat tone as a person enters deep sleep are clear and obvious factors contributing to OSA developing. But this explanation is also confounded by the presence of neck obesity.
Use of CPAP definitively primarily expands a collapsed upper airway, allowing for nasal breathing – and positive use of CPAP would prove that airway collapse is the cause of OSA.
Throat lesions, particularly enlarged tonsils, are well recognized as aggravators of OSA, and removal may provide full or partial or semi-permanent relief from OSA, which also indicates that enlarged tonsils may play a role in the pathogenesis of OSA.
Age
[edit]Old age is often accompanied by muscular and neurological loss of muscle tone of the upper airway. Decreased muscle tone is also temporarily caused by chemical depressants; alcoholic drinks and sedative medications being the most common. Permanent premature muscular tonal loss in the upper airway may be precipitated by traumatic brain injury, neuromuscular disorders, or poor adherence to chemical and or speech therapy treatments.
Muscle tone
[edit]Individuals with decreased muscle tone and increased soft tissue around the airway, and structural features that give rise to a narrowed airway are at high risk for OSA. Men, in which the anatomy is typified by increased mass in the torso and neck, are at increased risk of developing sleep apnea, especially through middle age and later. Typically, women experience this condition less frequently and to a lesser degree than do men, owing partially to physiology, but possibly also to differential levels of progesterone. Prevalence in post-menopausal women approaches that of men in the same age range. Women are at greater risk for developing OSA during pregnancy.[29]
Medication and lifestyle
[edit]Lifestyle factors such as smoking may also increase the chances of developing OSA as the chemical irritants in smoke tend to inflame the soft tissue of the upper airway and promote fluid retention, both of which can result in narrowing of the upper airway. Cigarettes may also have an impact due to a decline of blood nicotine levels, which alters sleep stability.[3] Smokers thus show a higher risk to develop OSA, but the effect of cigarettes on increased OSA is reversible with the cessation of smoking.[3] Children exposed to cigarette smoke may also develop OSA as the lymphadenoid tissue will proliferate excessively in contact with the irritants.[22] An individual may also experience or exacerbate OSA with the consumption of alcohol, sedatives, or any other medication that increases sleepiness as most of these drugs are also muscle relaxants.[30] Allergic rhinitis and asthma have also been shown to be implicated in the increased prevalence of adenotonsillar hypertrophy and OSA.[31][32]
Genetic
[edit]OSA also appears to have a genetic component; those with a family history of it are more likely to develop it themselves.
Craniofacial syndromes
[edit]Of substantial recent interest is the idea that there is a general human tendency towards developing short lower jaws (neoteny) is a major cause of OSA through a combined condition called glossoptosis. The posterior "normal" tongue is displaced backwards by a smaller "abnormal" anterior tongue and lower jaw. In much the same way, a narrow upper jaw will also contribute to OSA due to its relation to airway volume. A more narrow upper jaw results in more narrow nasal passages and a more narrow throat, this also appears to be why so many OSA patients experience nasal congestion especially while lying down.
Maxillofacial surgeons see many effects of small lower jaws, including crowded teeth, malocclusions, as well as OSA – all of which are treatable by surgical operations that increase and normalise jaw size. Operations such as custom BIMAX, GenioPaully, and IMDO (in adolescence) offer a valid medical option that replaces all traditional forms of OSA treatment – including CPAP, Mandibular Advancement Splints, tonsillectomy and UPPP. There are patterns of unusual facial features that occur in recognizable syndromes. Some of these craniofacial syndromes are genetic, others are from unknown causes. In many craniofacial syndromes, the features that are unusual involve the nose, mouth, and jaw, or resting muscle tone, and put the individual at risk for OSA syndrome.
Down syndrome is one such syndrome. In this chromosomal abnormality, several features combine to make the presence of obstructive sleep apnea more likely. The specific features of Down syndrome that predispose to obstructive sleep apnea include relatively low muscle tone, narrow nasopharynx, and large tongue. Obesity and enlarged tonsils and adenoids, conditions that occur commonly in the western population, are much more likely to be obstructive in a person with these features than without them. Obstructive sleep apnea does occur even more frequently in people with Down syndrome than in the general population. A little over 50% of all people with Down syndrome experience obstructive sleep apnea,[33] and some physicians advocate routine testing of this group.[34]
In other craniofacial syndromes, the abnormal feature may actually improve the airway, but its correction may put the person at risk for obstructive sleep apnea after surgery when it is modified. Cleft palate syndromes are such an example. During the newborn period, all humans are obligate nasal breathers. The palate is both the roof of the mouth and the floor of the nose. Having an open palate may make feeding difficult, but generally, does not interfere with breathing, in fact, if the nose is very obstructed, then an open palate may relieve breathing. There are a number of clefting syndromes in which the open palate is not the only abnormal feature; additionally, there is a narrow nasal passage – which may not be obvious. In such individuals, closure of the cleft palate – whether by surgery or by a temporary oral appliance – can cause the onset of obstruction.
Skeletal advancement in an effort to physically increase the pharyngeal airspace is often an option for craniofacial patients with upper airway obstruction and small lower jaws (mandibles). These syndromes include Treacher Collins syndrome and Pierre Robin sequence. Mandibular advancement surgery is one of the modifications needed to improve the airway, others may include reduction of the tongue, tonsillectomy or modified uvulopalatoplasty.[citation needed]
Post-operative complication
[edit]OSA can also occur as a serious post-operative complication that seems to be most frequently associated with pharyngeal flap surgery as compared to other procedures for the treatment of velopharyngeal inadequacy (VPI).[35] In OSA, recurrent interruptions of respiration during sleep are associated with temporary airway obstruction. Following pharyngeal flap surgery, depending on size and position, the flap itself may have an "obturator" or obstructive effect within the pharynx during sleep, blocking ports of airflow and hindering effective respiration.[36][37] There have been documented instances of severe airway obstruction, and reports of post-operative OSA continues to increase as healthcare professionals (i.e. physicians, speech language pathologists) become more educated about this possible dangerous condition.[38] Subsequently, in clinical practice, concerns of OSA have matched or exceeded interest in speech outcomes following pharyngeal flap surgery.[citation needed]
The surgical treatment for velopalatal insufficiency may cause obstructive sleep apnea syndrome. When velopalatal insufficiency is present, air leaks into the nasopharynx even when the soft palate should close off the nose. A simple test for this condition can be made by placing a tiny mirror on the nose, and asking the subject to say "P". This p sound, a plosive, is normally produced with the nasal airway closes off – all air comes out of the pursed lips, none from the nose. If it is impossible to say the sound without fogging a nasal mirror, there is an air leak – reasonable evidence of poor palatal closure. Speech is often unclear due to inability to pronounce certain sounds. One of the surgical treatments for velopalatal insufficiency involves tailoring the tissue from the back of the throat and using it to purposefully cause partial obstruction of the opening of the nasopharynx. This may actually cause OSA syndrome in susceptible individuals, particularly in the days following surgery, when swelling occurs (see below: Special Situation: Anesthesia and Surgery).[citation needed]
Finally, patients with OSA are at an increased risk of many perioperative complications when they are present for surgery, even if the planned procedure is not on the head and neck. Guidelines intended to reduce the risk of perioperative complications have been published.[39]
Consequences
[edit]There are 3 levels of consequences: physiologic, intermediate and clinical.[40] The physiologic consequences contain hypoxia, sleep fragmentation, autonomic nervous system dysregulation or hyperoxia.[40] The intermediate results regroup inflammation, pulmonary vasoconstriction, general metabolic dysfunction, oxidation of proteins and lipids or increased adiposity.[40] The clinical repercussions are composed by pulmonary hypertension, accidents, obesity, diabetes, different heart diseases or hypertension.[40]
In children
[edit]Obstructive sleep apnea is the most common Sleep-Disordered Breathing (SDB) and affects up to 11% of children born at term – it is even more common (3 to 6 times more) in children born pre-term.[41] As a SDB, OSA in children can lead to several adverse consequences, also in the long-term with consequences lasting into adulthood.[19] The implications of OSA in children are complex and cover a large scope of consequences: when it is left untreated, OSA can lead to morbidity affecting many different domains of life (organs, body systems, behavioral disturbance, depression, decreased quality of life, etc.).[22] Therefore, nocturnal symptoms indicating the presence of OSA (e.g. snoring, gasping, restless sleep and excessive energy used to breathe during sleep) are associated with daytime symptoms such as concentration and learning difficulties and irritability, neurocognitive development impairment, decreased school performance and behavioral difficulties.[19] For example, SDB such as OSA contributes to hyperactive behavior that can lead to the diagnosis and treatment of attention deficit hyperactivity disorder (ADHD). However, once the SDB is treated, the hyperactive behavior can improve, and the treatment can be stopped.[42] Obesity also has an impact on the consequences of OSA and lead to different manifestations or severity.[22] Studies have shown that, contrary to adults, children with obstructive sleep-disordered breathing are able to maintain cerebral oxygenation. However, the condition still has effects on the brain and can lead to adverse neurocognitive and behavioral sequelae. It is particularly concerning as those consequences happen while the brain is still developing.[41] The degree to which the sleep is disturbed and fragmented has been significantly linked to the severity of the consequences, the latter having the possibility to decrease once the sleep is improved.[21] It is more the disruption of sleep processes than the total amount of sleep the child experience that generates the adverse consequences on the child's daytime functioning;[21] it contributes to the hyperactivity for example.[42]
Children with OSA may experience learning and memory deficits, and OSA has been linked to lowered childhood IQ scores.[43] Untreated OSA can also cause children to not reach their height potential.[44]
Neurocognitive and behavioral consequences
[edit]Nocturnal sleep fragmentation has been linked to neurocognitive impairments, therefore, the identification of SDB such as OSA is crucial in children, those impairments having the possibility to be reversible with the appropriate treatment for the sleep disorder.[45] The neurocognitive and behavioral dysfunctions commonly present in children with OSA include the following: hyperactivity, impulsivity, aggressive behaviors,[22][21] low social and communication abilities and reduced adaptive skills.[19] Children with OSA commonly show cognitive deficits, resulting in attention and concentration difficulties, as well as lower academic performance and IQ.[19][21] Poor academic performances have been linked to OSA and suggested to result from cortical and sympathetic arousals and hypoxemia which affects memory consolidation.[46] A study with Indian children affected by OSA has shown poor school grades, including mathematics, science, language and physical education. This study allowed to see the overall impact of OSA on learning abilities associated with language or numeracy skills, and physical development.[46] It has been suggested that the deficits in academic performance related to OSA could be mediated through reduced executive functions or language skills,[47] those domains contributing highly to learning abilities and behavior. The deficits in school performance can nevertheless be improved if adenotonsillectomy is performed on children to treat the OSA.[47] It is thus crucial to identify the OSA for children with school difficulties; many cases remaining unnoticed.[47]
As studies have shown that learning skills and behaviors can be improved if the OSA is treated,[19][21] the neurocognitive and behavioral deficits are thus at least partly reversible.[22] This reversible dimension has been postulated to be negatively correlated to the duration of the symptoms, which would mean that the longer the OSA is left untreated, the less reversible are the consequences.[21]
Somatic and metabolic consequences
[edit]Similarly to adults, OSA in children is linked to a higher risk for cardiovascular morbidities,[22][21] due to increased sympathetic activity and impaired cardiac autonomic control.[41] Amongst the cardiovascular dysfunctions resulting from OSA, we can find systemic hypertension[21] and blood pressure dysregulation[19][48] (elevated blood pressure, or variability of the blood pressure for example[41]). The variability of the blood pressure has been shown to be correlated with the severity of the symptoms such as the frequency of the apnea and hypopnea.[48] Pulmonary hypertension is also common amongst the cardiovascular problems resulting from OSA.[19] Children with obstructive sleep-disordered breathing also show a faster heart rate during wakefulness and during sleep.[48]
In adult patients, OSA has been shown to be associated with insulin resistance.[49] In children, metabolic consequences of OSA are complicated to assess as they can also be associated to puberty and obesity (if present).[19] However, when OSA is associated with obesity, the interaction of the two conditions can lead to metabolic disturbances such as insulin resistance and altered lipidemia,[22] liver disease, abdominal adiposity and metabolic syndrome. Obesity interact with those effects.[48]
Nocturnal enuresis
[edit]Children with OSA also show a higher risk for nocturnal enuresis[19][50] and it is hypothesized to be caused by an excessive production of urine,[46][51] impaired performance of the bladder and urethra[52] or an inability to suppress the nocturnal bladder contraction, due to a failure to arouse.[51][52] The risk for nocturnal enuresis increases with the severity of the sleep-disordered breathing: the more respiratory events per hour of sleep, the higher is the risk for nocturnal enuresis.[52] Obesity may also play a role as it is associated with OSA and with nocturnal diuresis (due to unhealthy diet). The interaction between OSA and obesity might thus result in nocturnal enuresis.[51] Considering the high prevalence of nocturnal enuresis amongst children with sleep-disordered breathing, it is important to consider the latter in the differential diagnosis of nocturnal enuresis as the treatment of the sleep disorder might have a favourable therapeutic effect on the enuresis.[53][50][52] For example, an adenotonsillectomy performed to reduce OSA has a positive impact on nocturnal enuresis.[53][46] A study has shown that this surgery has 60–75% chance to resolve the nocturnal enuresis completely, and 80–85% chance to reduce its symptoms alongside others symptoms of OSA.[50]
Stunted growth
[edit]Untreated OSA in children can also lead to stunted growth. When sleep is disrupted in children that are still growing, there can be significant consequences. Human growth hormone (HGH) is secreted during the night, especially during deep, non-REM sleep. If this is disrupted, growth hormone secretion may be compromised; thus, growth may not occur normally and children may become shorter than their peers.[44]
Other consequences
[edit]Contrary to adults, excessive daytime sleepiness (EDS) is not the most commonly reported symptoms in children with OSA.[45] However, using objective questionnaires, it is possible to notice that the frequency of EDS in children is higher than what is reported by the parents or caretakers (40–50%).[21] And the risk for EDS is even increased when OSA is associated with obesity.[21][22]
Due to all the consequences and symptoms it generates, OSA in children lead to a significant decrease in the quality of life,[21] the decrease being even higher when obesity is present.[22] The quality of life can however be improved with the treatment of OSA.[22] SDB have also been linked to a higher rate of internalizing disorders such as anxiety and depression.[54] Indeed, depressive symptoms have shown to be higher in children with OSA,[54] especially in males.[55] Once again, the severity of depressive symptoms is positively correlated with the severity of the SDB.[54] It also interacts with obesity as obese children have higher risk to show depressive symptoms and obesity can cause OSA.[55] The link can also go the other way around with the depression inducing obesity (due to overeating) which worsens the OSA. Adenotonsillectomy can decrease the intensity of the depressive symptoms.[55]
Other consequences of a disturbed sleep in children with OSA comprise anhedonia[56][54] increased fatigue and decreased interest in daily activities, which in turn can affect the child's social relationships.[22]
In adults
[edit]While there are some similarities between adults and children, OSA does not have the same consequences in both populations.[57] Examples of similarities are the snoring – which is the most common complaint in both pediatric OSA and OSA in adults[57] – variability of blood pressure and cardiovascular morbidities.[3] A major difference is the excessive daytime sleepiness (EDS) which is commonly reported in adult OSA,[58] while it is not very common in pediatric OSA.[57] Nevertheless, OSA in adults also implies a large scope of adverse and serious consequences,[59] the latter leading to higher mortality amongst OSA patients.[60] Those consequences are even worsened by common morbidities such as obesity.[61]
Neurocognitive consequences
[edit]Similarly to children, OSA affects cognitive functions in adults.[57] Meta-analysis have shown that the most common cognitive impairments happen in the domains of attention, verbal and visual delayed long-term memory,[62] visuospatial/constructional abilities and executive functions,[63] such as mental flexibility.[62] The executive functioning – mainly dominated by the prefrontal cortex[64] – being significantly impaired in patients with OSA, it is believed that the prefrontal region and its connectivity are affected by sleep disorders.[65]
Regarding memory deficits, verbal memory is significantly impaired as patients show difficulties in recalling verbal information immediately as well as with a delay. While meta-analysis have shown no deficits in retention of information for patients with OSA, those impairment in verbal memory may be linked to problems in encoding information.[66] This deficit in encoding of information is also noticed in visuo-spatial memory; however, the visual memory seems to be intact in OSA patients.[66]
The cognitive impairments have been suggested to be resulting from sleep fragmentation and sleep deprivation, as well as the excessive daytime sleepiness associated with them.[63] More precisely, attention and memory deficits seem to result from sleep fragmentation, while deficits in global cognitive function (executive function, psychomotor function, language abilities) are more related to hypoxia or hypercarbia which accompanies the obstructive events during sleep.[63][67] However, no consistent correlation has been found between the degree of cognitive impairment and the severity of the sleep disturbance or hypoxia.[66][67] These impairments may improve with an effective treatment for OSA, such as continuous positive airway pressure (CPAP) therapy.[63] Driving a motor vehicle is an example of a complex task that relies on driver's cognitive abilities, such as attention, reaction time and vigilance.[68] Very brief moments of inattention called microsleep events could be an indicator for daytime vigilance impairment, although these may not be present in all drivers with obstructive sleep apnea.[69]
Behavioral consequences
[edit]The hyperactivity and difficulties in emotional regulation found in pediatric patients (children) are not reported in adults.[57] OSA in adults is nevertheless associated with personality changes and automatic behavior.[58] The biggest impact of OSA is the excessive daytime sleepiness (EDS), reported in approximately 30% of OSA patients.[70] EDS can be caused by the disturbance of sleep quality, the insufficient sleep duration or the sleep fragmentation[71] and it is responsible for further complications as it may lead to depressive symptoms,[72] impairments of social life and decreased effectiveness at work.[73][74] Studies have shown that those consequences of EDS can be improved following a CPAP treatment.[75]
Physiological and metabolic consequences
[edit]OSA in adults is associated with a higher risk for cardiovascular morbidities, diabetes, hypertension, coronary artery disease and stroke[57][58] – OSA might have a role in the etiology of these conditions. Those conditions may lead to increased mortality[57][58] that an appropriate treatment for OSA may reduce.[58] OSA is often linked with hypertension as it induces an increase in sympathetic activity that can lead to the elevation of blood pressure. The OSA-related hypercapnia has been suggested to be related to this development of hypertension.[76] Treating the OSA may prevent the development of hypertension.[77] The relationship between OSA and excess body weight is complex as obesity is more prevalent amongst OSA patients but can also be a risk factor for the development of OSA[58] – it accounts for 58% of adult cases.[78] Thus, both OSA and obesity (when present) may work synergistically and lead to hyperlipidemia, diabetes, insulin resistance and other symptoms of the metabolic syndrome.[3][58] The metabolic syndrome itself is often associated with OSA: 74–85% of OSA patients are diagnosed with it. CPAP therapy can lead to an improvement of some of the cardiovascular component of the metabolic syndrome[77] while weight loss is also recommended for its positive effects on OSA consequences and metabolic dysfunctions.[78] An intervention comprising exercise and diet is thus effective for the treatment of OSA as it positively impacts the severity of both obesity symptoms and OSA symptoms.[79]
Individuals with Type 2 diabetes are often co-diagnosed with OSA, where Type 2 diabetes prevalence rates range between 15% and 30% within the OSA population.[80] The relationship between OSA and Type 2 diabetes could possibly be explained by the fact that OSA-characteristic fragmented sleep and irregular hypoxemia leads to the dysregulated metabolism of glucose in the blood.[80] In particular, many polysomnography studies showed that OSA left untreated worsens glycemic control in individuals with Type 2 diabetes.[80] However, it is possible that the relationship between OSA and Type 2 diabetes is bidirectional since diabetes-related nerve dysfunction may affect the respiratory system and induce breathing disturbances during sleep.[80]
Psychological consequences
[edit]Sleep is of major importance for psychological and emotional health.[81] As it is greatly impaired in OSA, this condition is associated with mood disorders[82] and several psychological outcomes,[81] especially depression and anxiety.[83] Therefore, psychological disorders are commonly observed in OSA patients who show a higher prevalence of psychological distress, mostly due to the impaired sleep quality and structure and the repeated episodes of hypoxia.[81][84] The presence of psychological disorders may also worsen the sleep disorders which implies that the psychopathology may either be a factor or a consequence of the OSA.[83] The adverse consequences of OSA such as cardiovascular comorbidities and metabolic disturbances also play a role on the development of mental disorders,[83] and patients with chronic condition have also been reported to have higher risk to experience depression.[82] In OSA patients with psychiatric disorders, it is crucial to treat the OSA as it may reduce the psychiatric symptoms.[85]
Some cases of OSA are caused by nasal obstruction which has also been related to psychological problems due to an altered ratio of calcium and magnesium in brain cells. Nasal obstruction can thus aggravate the psychological health of OSA patients. Nasal surgery for those patients might decrease the OSA severity and improve the psychological symptoms.[84]
Other consequences
[edit]Untreated OSA also leads to a decreased quality of life, difficulties in social functioning,[58] occupational problems and accidents[65] and a greatly increased rate of vehicle accidents.[58][86][87] Those serious outcomes of OSA are mostly related to the excessive daytime sleepiness resulting from the sleep fragmentation and highlight the need to provide the patients with appropriate treatment.[58] Effective treatment majorly improves those adverse consequences, including quality of life.[58]
OSA patients also frequently report pain disorders such as headache or fibromyalgia, OSA patients showing an increased pain intensity alongside a decreased pain tolerance.[87]
Diagnosis
[edit]In a systematic review of published evidence, the United States Preventive Services Task Force in 2017 concluded that there was uncertainty about the accuracy or clinical utility of all potential screening tools for OSA,[88] and recommended that evidence is insufficient to assess the balance of benefits and harms of screening for OSA in asymptomatic adults.[89]
The diagnosis of OSA syndrome is made when the patient shows recurrent episodes of partial or complete collapse of the upper airway during sleep resulting in apneas or hypopneas, respectively.[90] Criteria defining an apnea or a hypopnea vary. The American Academy of Sleep Medicine (AASM) defines an apnea as a reduction in airflow of ≥ 90% lasting at least 10 seconds. A hypopnea is defined as a reduction in airflow of ≥ 30% lasting at least 10 seconds and associated with a ≥ 4% decrease in pulse oxygenation, or as a ≥ 30% reduction in airflow lasting at least 10 seconds and associated either with a ≥ 3% decrease in pulse oxygenation or with an arousal.[91]
To define the severity of the condition, the Apnea-Hypopnea Index (AHI) or the Respiratory Disturbance Index (RDI) are used. While the AHI measures the mean number of apneas and hypopneas per hour of sleep, the RDI adds to this measure the respiratory effort-related arousals (RERAs).[92] The OSA syndrome is thus diagnosed if the AHI is > 5 episodes per hour and results in daytime sleepiness and fatigue or when the RDI is ≥ 15 independently of the symptoms.[93] According to the American Association of Sleep Medicine, daytime sleepiness is determined as mild, moderate and severe depending on its impact on social life. Daytime sleepiness can be assessed with the Epworth Sleepiness Scale (ESS), a self-reported questionnaire on the propensity to fall asleep or doze off during daytime.[94] Screening tools for OSA itself comprise the STOP questionnaire, the Berlin questionnaire and the STOP-BANG questionnaire which has been reported as being a very powerful tool to detect OSA.[95][96]
Criteria
[edit]According to the International Classification of Sleep Disorders, there are 4 types of criteria. The first one concerns sleep – excessive sleepiness, nonrestorative sleep, fatigue or insomnia symptoms. The second and third criteria are about respiration – waking with breath holding, gasping, or choking; snoring, breathing interruptions or both during sleep. The last criterion revolved around medical issues as hypertension, coronary artery disease, stroke, heart failure, atrial fibrillation, type 2 diabetes mellitus, mood disorder or cognitive impairment. Two levels of severity are distinguished, the first one is determined by a polysomnography or home sleep apnea test demonstrating 5 or more predominantly obstructive respiratory events per hour of sleep and the higher levels are determined by 15 or more events. If the events are present less than 5 times per hour, no obstructive sleep apnea is diagnosed.[97]
A considerable night-to-night variability further complicates diagnosis of OSA. In unclear cases, multiple testing might be required to achieve an accurate diagnosis.[98]
Polysomnography
[edit]| Rating | AHI (adult) | AHI (pediatrics) |
|---|---|---|
| Normal | < 5 | <1 |
| Mild | ≥5, <15 | ≥1, <5 |
| Moderate | ≥15, <30 | ≥5, <10 |
| Severe | ≥ 30 | ≥10 |
Nighttime in-laboratory Level 1 polysomnography (PSG) is the gold standard test for diagnosis. Patients are monitored with EEG leads, pulse oximetry, temperature and pressure sensors to detect nasal and oral airflow, respiratory impedance plethysmography or similar resistance belts around the chest and abdomen to detect motion, an ECG lead, and EMG sensors to detect muscle contraction in the chin, chest, and legs. A hypopnea can be based on one of two criteria. It can either be a reduction in airflow of at least 30% for more than 10 seconds associated with at least 4% oxygen desaturation or a reduction in airflow of at least 30% for more than 10 seconds associated with at least 3% oxygen desaturation or an arousal from sleep on EEG.[99]
An "event" can be either an apnea, characterized by complete cessation of airflow for at least 10 seconds, or a hypopnea in which airflow decreases by 50 percent for 10 seconds or decreases by 30 percent if there is an associated decrease in the oxygen saturation or an arousal from sleep.[100] To grade the severity of sleep apnea, the number of events per hour is reported as the apnea-hypopnea index (AHI). For adults, an AHI of less than 5 is considered normal, an AHI of [5–15) is mild, [15–30) is moderate, and ≥30 events per hour characterizes severe sleep apnea. For pediatrics, an AHI of less than 1 is considered normal, an AHI of [1–5) is mild, [5–10) is moderate, and ≥10 events per hour characterizes severe sleep apnea.
Home oximetry
[edit]In patients who are at high likelihood of having OSA, a randomized controlled trial found that home oximetry (a non-invasive method of monitoring blood oxygenation) may be adequate and easier to obtain than formal polysomnography.[101] High probability patients were identified by an Epworth Sleepiness Scale (ESS) score of 10 or greater and a Sleep Apnea Clinical Score (SACS) of 15 or greater.[102] Home oximetry, however, does not measure apneic events or respiratory event-related arousals and thus does not produce an AHI value.
Treatment
[edit]Numerous treatment options are used in obstructive sleep apnea.[103] Avoiding alcohol and smoking is recommended,[104] as is avoiding medications that relax the central nervous system (for example, sedatives and muscle relaxants). Weight loss is recommended in those who are overweight. Continuous positive airway pressure (CPAP) and mandibular advancement devices are often used and found to be equally effective.[105][106] Physical training, even without weight loss, improves sleep apnea.[107] There is insufficient evidence to support widespread use of medications.[105] In selected patients, e.g. with tonsillar hyperplasia, tonsillectomy is recommended. In patients failing CPAP and oral appliances, surgical treatment with conservative uvulopalatopharyngoplasty (UPPP) as salvage surgery is recommended. Randomized controlled studies of the efficacy of UPPP are published, showing effect on nocturnal respiration and excessive daytime sleepiness,[108] and a systematic Meta-analysis.[109]
Physical intervention
[edit]The most widely used therapeutic intervention is positive airway pressure whereby a breathing machine pumps a controlled stream of air through a mask worn over the nose, mouth, or both. The additional pressure holds open the relaxed muscles. There are several variants:
- Continuous positive airway pressure (CPAP) is effective for both moderate and severe disease.[110] It is the most common treatment for obstructive sleep apnea.[111]
- Variable positive airway pressure (VPAP) (also known as bilevel (BiPAP or BPAP)) uses an electronic circuit to monitor the patient's breathing and provides two different pressures, a higher one during inhalation and a lower pressure during exhalation. This system is more expensive and is sometimes used with patients who have other coexisting respiratory problems or who find breathing out against an increased pressure to be uncomfortable or disruptive to their sleep.
- Nasal EPAP, which is a bandage-like device placed over the nostrils that utilizes a person's own breathing to create positive airway pressure to prevent obstructed breathing.[112]
- Automatic positive airway pressure, also known as "Auto CPAP", incorporates pressure sensors and monitors the person's breathing.[113][114]
- A 5% reduction in weight among those with moderate to severe OSA may decrease symptoms similarly to CPAP.[115]
Encouraging people with moderate to severe OSA to use CPAP devices can be challenging as their use often requires a behavioural change in sleeping habits.[111] 8% of people who use CPAP devices stop using them after the first night, and 50% of people with moderate to severe OSA stop using their devices in the first year.[111] Educational initiatives and supportive interventions to help improve compliance with CPAP therapy have been shown to improve the length of time people who need CPAP therapy use their devices.[111]
Oral appliances or splints are often preferred but may not be as effective as CPAP.[110] This device is a mouthguard similar to those used in sports to protect the teeth. It is designed to hold the lower jaw slightly down and forward relative to the natural, relaxed position. This position holds the tongue farther away from the back of the airway and may be enough to relieve apnea or improve breathing.
Many people benefit from sleeping at a 30-degree elevation of the upper body[116] or higher, as if in a recliner. Doing so helps prevent the gravitational collapse of the airway. Sleeping on a side as opposed to sleeping on the back is also recommended.[117][118][119]
Some studies have suggested that playing a wind instrument may reduce snoring and apnea incidents.[120] This may be especially true of double reed instruments.[121]
Rapid Palatal Expansion
[edit]In children, orthodontic treatment to expand the volume of the nasal airway, such as nonsurgical Rapid Palatal expansion is common.
Since the palatal suture is fused in adults, regular RPE using tooth-borne expanders cannot be performed. Mini-implant assisted rapid palatal expansion (MARPE) has been recently developed as a non-surgical option for the transverse expansion of the maxilla in adults. This method increases the volume of the nasal cavity and nasopharynx, leading to increased airflow and reduced respiratory arousals during sleep.[122][123] Changes are permanent with minimal complications.
Surgery
[edit]Surgical treatments to modify airway anatomy, known as sleep surgery, are varied and must be tailored to the specific airway obstruction needs of a patient. Surgery is not considered a first line treatment for obstructive sleep apnea in adults. There are prospective, randomized, comparative clinical trials,[124] and also a systematic Meta-analysis,[109] showing evidence that conservative uvulopalatopharyngoplasty (UPPP) with or without tonsillectomy is effective in selected patients failing conservative treatment. For those with obstructive sleep apnea unable or unwilling to comply with first line treatment, the surgical intervention has to be adapted to an individual's specific anatomy and physiology, personal preference and disease severity.[103] Uvulopalatopharyngoplasty with or without is the most common surgery for patients with obstructive sleep apnea. Studies have shown that treatment effect of tonsillectomy increases with tonsil size.[125] However, there is little randomized clinical trial evidence for other types of sleep surgery.[105]
There are a number of different operations that may be performed:
- Septoplasty is a corrective surgical procedure for Nasal septum deviation in which the nasal septum is straightened.
- Tonsillectomy[125] or adenoidectomy in an attempt to increase the size of the airway.
- Removal or reduction of parts of the soft palate and some or all of the uvula, such as uvulopalatopharyngoplasty (UPPP) or laser-assisted uvulopalatoplasty (LAUP). Modern variants of this procedure sometimes use radiofrequency waves to heat and remove tissue.
- Turbinectomy is a surgical procedure in which all or some of the turbinate bones are removed to relieve nasal obstruction.
- Reduction of the tongue base, either with laser excision or radiofrequency ablation.
- Genioglossus advancement, in which a small portion of the lower jaw that attaches to the tongue is moved forward, to pull the tongue away from the back of the airway.
- Hyoid suspension, in which the hyoid bone in the neck, another attachment point for tongue muscles, is pulled forward in front of the larynx.
- Maxillomandibular advancement[126]
In the morbidly obese, a major loss of weight (such as what occurs after bariatric surgery) can sometimes cure the condition.
OSA in children is often due to enlarged tonsils and adenoids because the lymphoid tissue grows fast during young age. Surgical removal of enlarged tonsils (tonsillectomy) and the adenoid (adenoidectomy) are first line treatment among children with OSA. The operation is a common procedure but in the most extreme cases, children with severe OSA requires special precautions before, surgery (see "Surgery and obstructive sleep apnea syndrome" below). In some countries, a milder surgical procedure called tonsillotomy is used to remove the protruding tonsillar tissue, a method associated with less pain and lower risk of postoperative hemorrhage.[127]
Neurostimulation
[edit]For patients who cannot use a continuous positive airway pressure device, the U.S. Food and Drug Administration in 2014 granted pre-market approval for an upper airway stimulation system that senses respiration and delivers mild electrical stimulation to the hypoglossal nerve in order to increase muscle tone at the back of the tongue so it will not collapse over the airway. The device includes a handheld patient controller to allow it to be switched on before sleep and is powered by an implantable pulse generator, similar to one used for cardiac rhythm management. Approval for this active implantable neuromodulation device was preceded by a clinical trial whose results were published in the New England Journal of Medicine.[128][129]
Radiofrequency ablation
[edit]Radiofrequency ablation (RFA), which is conceptually analogous in some ways to surgery, uses low frequency (300 kHz to 1 MHz)[130] radio wave energy to target tissue, causing coagulative necrosis. RFA achieves its effects at 40 °C to 70 °C[131] unlike other electrosurgical devices which require 400 °C to 600 °C for efficacy.[132]
Subsequent evaluations of safety and efficacy have led to the recognition of RFA by the American Academy of Otolaryngology[133] as a somnoplasty treatment option in selected situations for mild to moderate OSA, but the evidence was judged insufficient for routine adoption by the American College of Physicians.[105]
RFA has some potential advantages in carefully selected medical settings, such as intolerance to the CPAP device. For example, when adherence is defined as greater than four hours of nightly use, 46% to 83% of patients with obstructive sleep apnea are non-adherent with CPAP[134] for a variety of reasons, including discomfort while sleeping.
RFA is usually performed in an outpatient setting, using either local anesthetics or conscious sedation anesthesia, the procedure itself typically lasting under 3 minutes. The targeted tissue, such as tongue or palate, is usually approached through the mouth without the need for incisions, although occasionally the target is approached through the neck using assisted imaging.[135] If the tongue is being targeted, this can be done from either dorsal or ventral side. Complications include ulceration, infection, nerve weakness or numbness and swelling. These complications occur in less than 1% of procedures.[130]
Medications
[edit]Evidence is insufficient to support the use of medications to treat obstructive sleep apnea directly.[105][136][137] This includes the use of fluoxetine, paroxetine, acetazolamide, and tryptophan among others.[105][138]
Recent studies are trying to investigate cannabinoids as a treatment for OSA, especially dronabinol which is a synthetic form of THC (delta-9-tetrahydrocannabinol). Cannabis is known to influence sleep, for example it can reduce sleep onset latency, however, results are not consistent.[139] Studies about dronabinol have shown positive impact on the OSA, as they observed a reduced AHI (Apnea-Hypopnea Index) and an increased self-reported sleepiness (the objective sleepiness being unaffected).[140] However, more evidence are needed as many effects of those substances remain unknown, especially the effects of a long-term intake.[141] The effect on sleepiness and weight gain are particularly of concern.[142] Because of uncertainty about its effects and a lack of consistent evidence, the American Academy of Sleep Medicine does not approve the use of medical cannabis for the treatment of OSA.[141][143]
However in two phase 3, double-blind, randomized, controlled trials involving adults with moderate-to-severe obstructive sleep apnea and obesity, tirzepatide for 52 weeks substantially reduced apneic-hypopnic events, body weight, hypoxic burden, hsCRP concentration, and systolic blood pressure, and it improved sleep-related patient-reported outcomes, all presumably mediated via induced weight loss of 18–20% from baseline.[144]
There are multiple drugs approved for managing the excessive daytime sleepiness associated with OSA but not the underlying cause. These include solriamfetol, modafinil, and armodafinil.[145][146][147]
Oral appliances / functional orthopedic appliances
[edit]Evidence to support oral appliances/functional orthopedic appliances in children is insufficient with very low evidence of effect. However, the oral appliances/functional orthopedic appliances may be considered in specified cases as an auxiliary in the treatment of children who have craniofacial anomalies which are risk factors of apnea.[148]
Prognosis
[edit]Stroke and other cardiovascular diseases are related to OSA, and those under the age of 70 have an increased risk of early death.[90] Persons with sleep apnea have a 30% higher risk of heart attack or death than those unaffected.[149] In severe and prolonged cases, increased in pulmonary pressures are transmitted to the right side of the heart. This can result in a severe form of congestive heart failure known as cor pulmonale. Diastolic function of the heart also becomes affected.[150] Elevated arterial pressure (i.e., hypertension) can be a consequence of OSA syndrome.[151] When hypertension is caused by OSA, it is distinctive in that, unlike most cases (so-called essential hypertension), the readings do not drop significantly when the individual is sleeping (non-dipper) or even increase (inverted dipper).[152]
Without treatment, the sleep deprivation and lack of oxygen caused by sleep apnea increases health risks such as cardiovascular disease, aortic disease (e.g. aortic aneurysm),[153] high blood pressure,[154][155] stroke,[156] diabetes, clinical depression,[157] weight gain, obesity,[40] and even death.
OSA is associated with cognitive impairment, including deficits in inductive and deductive reasoning, attention, vigilance, learning, executive functions, and episodic and working memory. OSA is associated with increased risk for developing mild cognitive impairment and dementia, and has been associated with neuroanatomical changes (reductions in volumes of the hippocampus, and gray matter volume of the frontal and parietal lobes) which can however be at least in part reversed with CPAP treatment.[158][159]
Epidemiology
[edit]Until the 1990s, little was known regarding the frequency of OSA.[160] A recent meta-analysis of 24 epidemiological studies on the prevalence of OSA in the general population aged 18 and older revealed that for ≥ 5 apnea events per hour, OSA prevalence ranged from 9% to 38%, specifically ranging from 13% to 33% in men and 6% to 19% in women, while in the population aged 65 and older, OSA prevalence was as high as 84%, including 90% in men and 78% in women.[161] Nevertheless, for ≥ 15 apnea events per hour, OSA prevalence ranged from 6% to 17%, and almost 49% prevalence in the older population aged 65 and older.[161] Moreover, a higher BMI is also linked to a higher prevalence of OSA, where a 10% increase in body weight led to a 6-fold risk of OSA in obese men and women.[161]
However, OSA is underdiagnosed as it is not always accompanied by daytime sleepiness which can leave the sleep-disordered breathing unnoticed.[82] The prevalence of OSA with daytime sleepiness is thus estimated to affect 3% to 7% of men and 2% to 5% of women, and the disease is common in both developed and developing countries.[160] OSA prevalence increases with age and is most commonly diagnosed in individuals over 65 years old, with estimations ranging from 22.1% to 83.6%.[161] The prevalence has drastically increased in recent decades due to the incidence of obesity.[77]
Men are more affected by OSA than women, but the phenomenology differs between both genders.[40] Snoring and witnessed apnea are more frequent among men but insomnia for example is more frequent among women.[40] The OSA frequency increase with age for the women.[40] The mortality is higher for women.[40]
Some studies report that it is more frequent among the Hispanic and African American population than among the white population.[40]
If studied carefully in a sleep lab by polysomnography (formal "sleep study"), it is believed that approximately 1 in 5 American adults would have at least mild OSA.[162]
Research
[edit]Neurostimulation is being studied as a method of treatment.[163] An implanted hypoglossal nerve stimulation system received European CE Mark (Conformité Européenne) approval in March 2012.[164] Also being studied are exercises of the muscles around the mouth and throat through activities such as playing the didgeridoo.[165][166]
See also
[edit]References
[edit]- ^ Punjabi, Naresh M.; Caffo, Brian S.; Goodwin, James L.; Gottlieb, Daniel J.; Newman, Anne B.; O'Connor, George T.; Rapoport, David M.; Redline, Susan; Resnick, Helaine E.; Robbins, John A.; Shahar, Eyal; Unruh, Mark L.; Samet, Jonathan M. (18 August 2009). "Sleep-Disordered Breathing and Mortality: A Prospective Cohort Study". PLOS Medicine. 6 (8): e1000132. doi:10.1371/journal.pmed.1000132. PMC 2722083. PMID 19688045.
- ^ Barnes L, ed. (2009). Surgical pathology of the head and neck (3rd ed.). New York: Informa healthcare. ISBN 978-1-4200-9163-2.: 226
- ^ a b c d e Young, Terry; Skatrud, J; Peppard, PE (28 April 2004). "Risk Factors for Obstructive Sleep Apnea in Adults". JAMA. 291 (16): 2013–2016. doi:10.1001/jama.291.16.2013. PMID 15113821. S2CID 12315855.
- ^ Berry, Richard B.; Budhiraja, Rohit; Gottlieb, Daniel J.; Gozal, David; Iber, Conrad; Kapur, Vishesh K.; Marcus, Carole L.; Mehra, Reena; Parthasarathy, Sairam; Quan, Stuart F.; Redline, Susan; Strohl, Kingman P.; Ward, Sally L. Davidson; Tangredi, Michelle M.; American Academy of Sleep Medicine (2012). "Rules for Scoring Respiratory Events in Sleep: Update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events". Journal of Clinical Sleep Medicine. 08 (5): 597–619. doi:10.5664/jcsm.2172. PMC 3459210. PMID 23066376.
- ^ American Academy of Sleep Medicine (2014). International Classification of Sleep Disorders, 3rd edition. Darien, IL: American Academy of Sleep Medicine.
- ^ Tung P, Levitzky YS, Wang R, Weng J, Quan SF, Gottlieb DJ, et al. (July 2017). "Obstructive and Central Sleep Apnea and the Risk of Incident Atrial Fibrillation in a Community Cohort of Men and Women". Journal of the American Heart Association. 6 (7). doi:10.1161/jaha.116.004500. PMC 5586257. PMID 28668820.
- ^ Stoohs RA, Blum HC, Knaack L, Butsch-von-der-Heydt B, Guilleminault C (March 2005). "Comparison of pleural pressure and transcutaneous diaphragmatic electromyogram in obstructive sleep apnea syndrome". Sleep. 28 (3): 321–329. PMID 16173653.
- ^ a b American Academy of Sleep Medicine. "AASM Practice Guidelines". AASM. Retrieved 19 June 2019.
- ^ Mbata, GC; Chukwuka, JC (2012). "Obstructive Sleep Apnea Hypopnea Syndrome". Annals of Medical and Health Sciences Research. 2 (1): 74–77. doi:10.4103/2141-9248.96943. PMC 3507119. PMID 23209996.
- ^ Caporale, Mina; Palmeri, Rosanna; Corallo, Francesco; Muscarà, Nunzio; Romeo, Laura; Bramanti, Alessia; Marino, Silvia; Lo Buono, Viviana (23 May 2020). "Cognitive impairment in obstructive sleep apnea syndrome: a descriptive review". Sleep and Breathing. 25 (1): 29–40. doi:10.1007/s11325-020-02084-3. PMID 32447633. S2CID 218835830.
- ^ Ezzeddini R, Darabi M, Ghasemi B, Jabbari Moghaddam Y, Abdollahi SH, Rashtchizadeh N, Gharahdaghi A, Darabi M, Ansarin M, Shaaker M, Samadi A, Karamravan J (Apr 2012). "Circulating phospholipase-A2 activity in obstructive sleep apnea and recurrent tonsillitis". Int J Pediatr Otorhinolaryngol. 76 (4): 741–744. doi:10.1016/j.ijporl.2011.12.026. PMID 22297210.
- ^ Ezzedini R, Darabi M, Ghasemi B, Darabi M, Fayezi SH, Jabbari Moghaddam Y, Mehdizadeh A, Abdollahi SH, Gharahdaghi A (May 2013). "Tissue fatty acid composition in obstructive sleep apnea and recurrent tonsillitis". Int J Pediatr Otorhinolaryngol. 77 (6): 1008–1012. doi:10.1016/j.ijporl.2013.03.033. PMID 23643333.
- ^ "Obstructive Sleep Apnea". The Lecturio Medical Concept Library. 7 August 2020. Retrieved 21 August 2021.
- ^ Gale, Shawn D.; Hopkins, Ramona O. (January 2004). "Effects of hypoxia on the brain: Neuroimaging and neuropsychological findings following carbon monoxide poisoning and obstructive sleep apnea". Journal of the International Neuropsychological Society. 10 (1): 60–71. doi:10.1017/S1355617704101082. PMID 14751008. S2CID 23745738.
- ^ Henry, Doug; Rosenthal, Leon (February 2013). "'Listening for his breath:' The significance of gender and partner reporting on the diagnosis, management, and treatment of obstructive sleep apnea". Social Science & Medicine. 79: 48–56. doi:10.1016/j.socscimed.2012.05.021. PMID 22770968.
- ^ O'Brien, Louise M.; Holbrook, Cheryl R.; Mervis, Carolyn B.; Klaus, Carrie J.; Bruner, Jennifer L.; Raffield, Troy J.; Rutherford, Jennifer; Mehl, Rochelle C.; Wang, Mei; Tuell, Andrew; Hume, Brittany C.; Gozal, David (March 2003). "Sleep and Neurobehavioral Characteristics of 5- to 7-Year-Old Children With Parentally Reported Symptoms of Attention-Deficit/Hyperactivity Disorder". Pediatrics. 111 (3): 554–563. doi:10.1542/peds.111.3.554. PMID 12612236.
- ^ Schechter, Michael S. (April 2002). "Technical Report: Diagnosis and Management of Childhood Obstructive Sleep Apnea Syndrome". Pediatrics. 109 (4): e69. doi:10.1542/peds.109.4.e69. PMID 11927742.
- ^ Rosen, Carol L.; Storfer-Isser, Amy; Taylor, H. Gerry; Kirchner, H. Lester; Emancipator, Judith L.; Redline, Susan (December 2004). "Increased Behavioral Morbidity in School-Aged Children With Sleep-Disordered Breathing". Pediatrics. 114 (6): 1640–1648. doi:10.1542/peds.2004-0103. PMID 15574628. S2CID 7655711.
- ^ a b c d e f g h i j k l Dehlink E, Tan HL (February 2016). "Update on paediatric obstructive sleep apnoea". Journal of Thoracic Disease. 8 (2): 224–235. doi:10.3978/j.issn.2072-1439.2015.12.04. PMC 4739955. PMID 26904263.
- ^ Jazi SM, Barati B, Kheradmand A (December 2011). "Treatment of adenotonsillar hypertrophy: A prospective randomized trial comparing azithromycin vs. fluticasone". Journal of Research in Medical Sciences. 16 (12): 1590–1597. PMC 3434901. PMID 22973368.
- ^ a b c d e f g h i j k l m n Capdevila OS, Kheirandish-Gozal L, Dayyat E, Gozal D (February 2008). "Pediatric obstructive sleep apnea: complications, management, and long-term outcomes". Proceedings of the American Thoracic Society. 5 (2): 274–282. doi:10.1513/pats.200708-138MG. PMC 2645258. PMID 18250221.
- ^ a b c d e f g h i j k l m n o Dayyat E, Kheirandish-Gozal L, Gozal D (September 2007). "Childhood Obstructive Sleep Apnea: One or Two Distinct Disease Entities?". Sleep Medicine Clinics. 2 (3): 433–444. doi:10.1016/j.jsmc.2007.05.004. PMC 2084206. PMID 18769509.
- ^ Kalra M, Inge T, Garcia V, Daniels S, Lawson L, Curti R, et al. (July 2005). "Obstructive sleep apnea in extremely overweight adolescents undergoing bariatric surgery". Obesity Research. 13 (7): 1175–1179. doi:10.1038/oby.2005.139. PMID 16076986.
- ^ a b Verhulst SL, Franckx H, Van Gaal L, De Backer W, Desager K (June 2009). "The effect of weight loss on sleep-disordered breathing in obese teenagers". Obesity. 17 (6): 1178–1183. doi:10.1038/oby.2008.673. PMID 19265797. S2CID 205526401.
- ^ Bower CM, Gungor A (February 2000). "Pediatric obstructive sleep apnea syndrome". Otolaryngologic Clinics of North America. 33 (1): 49–75. doi:10.1016/s0030-6665(05)70207-3. PMID 10637344.
- ^ Mills, Jacqueline; Kuohung, Wendy (2019). "Impact of circadian rhythms on female reproduction and infertility treatment success". Current Opinion in Endocrinology, Diabetes & Obesity. 26 (6): 317–321. doi:10.1097/MED.0000000000000511. PMID 31644470. S2CID 204865937.
- ^ Saboisky JP, Butler JE, Gandevia SC, Eckert DJ (15 June 2012). "Functional role of neural injury in obstructive sleep apnea". Frontiers in Neurology. 3: 95. doi:10.3389/fneur.2012.00095. PMC 3375463. PMID 22715333.
- ^ Schwab RJ, Kim C, Bagchi S, Keenan BT, Comyn FL, Wang S, et al. (June 2015). "Understanding the anatomic basis for obstructive sleep apnea syndrome in adolescents". American Journal of Respiratory and Critical Care Medicine. 191 (11): 1295–1309. doi:10.1164/rccm.201501-0169OC. PMC 4476519. PMID 25835282.
- ^ Edwards N, Sullivan CE (2008). "Sleep-Disordered Breathing in Pregnancy". Sleep Medicine Clinics. 3 (6): 81–95. doi:10.1016/j.jsmc.2007.10.010. PMC 1746354. PMID 12037233.
- ^ "Sleep apnea – Symptoms and causes". Mayo Clinic.
- ^ Lu LR, Peat JK, Sullivan CE (August 2003). "Snoring in preschool children: prevalence and association with nocturnal cough and asthma". Chest. 124 (2): 587–593. doi:10.1378/chest.124.2.587. PMID 12907547.
- ^ McColley, Susanna A.; Carroll, John L.; Curtis, Shelly; Loughlin, Gerald M.; Sampson, Hugh A. (January 1997). "High Prevalence of Allergic Sensitization in Children With Habitual Snoring and Obstructive Sleep Apnea". Chest. 111 (1): 170–173. doi:10.1378/chest.111.1.170. PMID 8996012.
- ^ de Miguel-Díez J, Villa-Asensi JR, Alvarez-Sala JL (December 2003). "Prevalence of sleep-disordered breathing in children with Down syndrome: polygraphic findings in 108 children". Sleep. 26 (8): 1006–1009. doi:10.1093/sleep/26.8.1006. PMID 14746382.
- ^ Shott SR, Amin R, Chini B, Heubi C, Hotze S, Akers R (April 2006). "Obstructive sleep apnea: Should all children with Down syndrome be tested?". Archives of Otolaryngology–Head & Neck Surgery. 132 (4): 432–436. doi:10.1001/archotol.132.4.432. PMID 16618913.
- ^ Sloan, Gerald M. (March 2000). "Posterior Pharyngeal Flap and Sphincter Pharyngoplasty: The State of the Art". The Cleft Palate-Craniofacial Journal. 37 (2): 112–122. doi:10.1597/1545-1569_2000_037_0112_ppfasp_2.3.co_2. PMID 10749049. S2CID 208150617.
- ^ Pugh, M.B. et al. (2000). Apnea. Stedman's Medical Dictionary (27th ed.) Retrieved June 18, 2006 from STAT!Ref Online Medical Library database.[page needed]
- ^ Liao YF, Noordhoff MS, Huang CS, Chen PK, Chen NH, Yun C, Chuang ML (March 2004). "Comparison of obstructive sleep apnea syndrome in children with cleft palate following Furlow palatoplasty or pharyngeal flap for velopharyngeal insufficiency". The Cleft Palate-Craniofacial Journal. 41 (2): 152–156. doi:10.1597/02-162. PMID 14989690. S2CID 20976608.
- ^ McWilliams BJ, Peterson-Falzone SJ, Hardin-Jones MA, Karnell MP (2001). Cleft palate speech (3rd ed.). St. Louis: Mosby. ISBN 978-0-8151-3153-3.[page needed]
- ^ Gross JB, Bachenberg KL, Benumof JL, Caplan RA, Connis RT, Coté CJ, et al. (May 2006). "Practice guidelines for the perioperative management of patients with obstructive sleep apnea: a report by the American Society of Anesthesiologists Task Force on Perioperative Management of patients with obstructive sleep apnea". Anesthesiology. 104 (5): 1081–1093, quiz 1117–1118. doi:10.1097/00000542-200605000-00026. PMID 16645462. S2CID 14151958.
- ^ a b c d e f g h i j Foldvary-Schaefer, Nancy R.; Waters, Tina E. (August 2017). "Sleep-Disordered Breathing". Continuum: Lifelong Learning in Neurology. 23 (4): 1093–1116. doi:10.1212/01.CON.0000522245.13784.f6. PMID 28777178. S2CID 20852866.
- ^ a b c d Walter LM, Horne RS (2018). "Obstructive Sleep-Disordered Breathing in Children; impact on the Developing Brain". Pediatric Respirology and Critical Care Medicine. 2 (4): 58–64. doi:10.4103/prcm.prcm_16_18. S2CID 80862031.
- ^ a b Chervin RD, Archbold KH (May 2001). "Hyperactivity and polysomnographic findings in children evaluated for sleep-disordered breathing". Sleep. 24 (3): 313–320. doi:10.1093/sleep/24.3.313. PMID 11322714.
- ^ Halbower, Ann C; Degaonkar, Mahaveer; Barker, Peter B; Earley, Christopher J; Marcus, Carole L; Smith, Philip L; Prahme, M. Cristine; Mahone, E. Mark (2006). "Childhood Obstructive Sleep Apnea Associates with Neuropsychological Deficits and Neuronal Brain Injury". PLOS Medicine. 3 (8): e301. doi:10.1371/journal.pmed.0030301. PMC 1551912. PMID 16933960.
- ^ a b Nieminen P, Löppönen T, Tolonen U, Lanning P, Knip M, Löppönen H. Growth and biochemical markers of growth in children with snoring and obstructive sleep apnea. Pediatrics. 2002;109(4):e55. doi:10.1542/peds.109.4.e55
- ^ a b O'Brien LM, Mervis CB, Holbrook CR, Bruner JL, Smith NH, McNally N, et al. (June 2004). "Neurobehavioral correlates of sleep-disordered breathing in children". Journal of Sleep Research. 13 (2): 165–172. doi:10.1111/j.1365-2869.2004.00395.x. PMID 15175097. S2CID 25860827.
- ^ a b c d Goyal A, Pakhare AP, Bhatt GC, Choudhary B, Patil R (2018). "Association of pediatric obstructive sleep apnea with poor academic performance: A school-based study from India". Lung India. 35 (2): 132–136. doi:10.4103/lungindia.lungindia_218_17. PMC 5846262. PMID 29487248.
- ^ a b c Galland B, Spruyt K, Dawes P, McDowall PS, Elder D, Schaughency E (October 2015). "Sleep Disordered Breathing and Academic Performance: A Meta-analysis". Pediatrics. 136 (4): e934–946. doi:10.1542/peds.2015-1677. PMID 26347434. S2CID 20590026.
- ^ a b c d Amin R, Somers VK, McConnell K, Willging P, Myer C, Sherman M, et al. (January 2008). "Activity-adjusted 24-hour ambulatory blood pressure and cardiac remodeling in children with sleep disordered breathing". Hypertension. 51 (1): 84–91. doi:10.1161/HYPERTENSIONAHA.107.099762. PMID 18071053. S2CID 1736152.
- ^ Ip, Mary S. M.; Lam, Bing; Ng, Matthew M. T.; Lam, Wah Kit; Tsang, Kenneth W. T.; Lam, Karen S. L. (1 March 2002). "Obstructive Sleep Apnea Is Independently Associated with Insulin Resistance". American Journal of Respiratory and Critical Care Medicine. 165 (5): 670–676. doi:10.1164/ajrccm.165.5.2103001. PMID 11874812.
- ^ a b c Basha, Suzanne; Bialowas, Christie; Ende, Kevin; Szeremeta, Wasyl (2005). "Effectiveness of Adenotonsillectomy in the Resolution of Nocturnal Enuresis Secondary to Obstructive Sleep Apnea". The Laryngoscope. 115 (6): 1101–1103. doi:10.1097/01.MLG.0000163762.13870.83. PMID 15933530. S2CID 44686421.
- ^ a b c Barone JG, Hanson C, DaJusta DG, Gioia K, England SJ, Schneider D (July 2009). "Nocturnal enuresis and overweight are associated with obstructive sleep apnea". Pediatrics. 124 (1): e53–59. doi:10.1542/peds.2008-2805. PMID 19564269. S2CID 7324455.
- ^ a b c d Brooks, Lee J.; Topol, Howard I. (May 2003). "Enuresis in children with sleep apnea". The Journal of Pediatrics. 142 (5): 515–518. doi:10.1067/mpd.2003.158. PMID 12756383.
- ^ a b Weissbach A, Leiberman A, Tarasiuk A, Goldbart A, Tal A (August 2006). "Adenotonsilectomy improves enuresis in children with obstructive sleep apnea syndrome". International Journal of Pediatric Otorhinolaryngology. 70 (8): 1351–1356. doi:10.1016/j.ijporl.2006.01.011. PMID 16504310.
- ^ a b c d Carotenuto M, Esposito M, Parisi L, Gallai B, Marotta R, Pascotto A, Roccella M (2012). "Depressive symptoms and childhood sleep apnea syndrome". Neuropsychiatric Disease and Treatment. 8: 369–373. doi:10.2147/NDT.S35974. PMC 3430390. PMID 22977304.
- ^ a b c Yilmaz E, Sedky K, Bennett DS (November 2013). "The relationship between depressive symptoms and obstructive sleep apnea in pediatric populations: a meta-analysis". Journal of Clinical Sleep Medicine. 9 (11): 1213–1220. doi:10.5664/jcsm.3178. PMC 3805811. PMID 24235907.
- ^ Crabtree VM, Varni JW, Gozal D (September 2004). "Health-related quality of life and depressive symptoms in children with suspected sleep-disordered breathing". Sleep. 27 (6): 1131–1138. doi:10.1093/sleep/27.6.1131. PMID 15532207.
- ^ a b c d e f g Alsubie HS, BaHammam AS (January 2017). "Obstructive Sleep Apnoea: Children are not little Adults". Paediatric Respiratory Reviews. 21: 72–79. doi:10.1016/j.prrv.2016.02.003. PMID 27262609.
- ^ a b c d e f g h i j k McNicholas WT (February 2008). "Diagnosis of obstructive sleep apnea in adults". Proceedings of the American Thoracic Society. 5 (2): 154–160. doi:10.1513/pats.200708-118MG. PMID 18250207.
- ^ Stranks EK, Crowe SF (March 2016). "The Cognitive Effects of Obstructive Sleep Apnea: An Updated Meta-analysis". Archives of Clinical Neuropsychology. 31 (2): 186–193. doi:10.1093/arclin/acv087. PMID 26743325.
- ^ Robichaud-Hallé L, Beaudry M, Fortin M (September 2012). "Obstructive sleep apnea and multimorbidity". BMC Pulmonary Medicine. 12 (60): 60. doi:10.1186/1471-2466-12-60. PMC 3515504. PMID 23006602.
- ^ Romero-Corral A, Caples SM, Lopez-Jimenez F, Somers VK (March 2010). "Interactions between obesity and obstructive sleep apnea: implications for treatment". Chest. 137 (3): 711–719. doi:10.1378/chest.09-0360. PMC 3021364. PMID 20202954.
- ^ a b Fulda S, Schulz H (2003). "Cognitive Dysfunction in Sleep-Related Breathing Disorders: A Meta-Analysis". Sleep Research Online. 5 (1): 19–51.
- ^ a b c d Bucks RS, Olaithe M, Eastwood P (January 2013). "Neurocognitive function in obstructive sleep apnoea: a meta-review". Respirology. 18 (1): 61–70. doi:10.1111/j.1440-1843.2012.02255.x. PMID 22913604. S2CID 27228461.
- ^ Nejati V, Salehinejad MA, Nitsche MA (January 2018). "Interaction of the Left Dorsolateral Prefrontal Cortex (l-DLPFC) and Right Orbitofrontal Cortex (OFC) in Hot and Cold Executive Functions: Evidence from Transcranial Direct Current Stimulation (tDCS)". Neuroscience. 369: 109–123. doi:10.1016/j.neuroscience.2017.10.042. PMID 29113929. S2CID 207258425.
- ^ a b Beebe DW, Groesz L, Wells C, Nichols A, McGee K (May 2003). "The neuropsychological effects of obstructive sleep apnea: a meta-analysis of norm-referenced and case-controlled data". Sleep. 26 (3): 298–307. doi:10.1093/sleep/26.3.298. PMID 12749549.
- ^ a b c Wallace A, Bucks RS (February 2013). "Memory and obstructive sleep apnea: a meta-analysis". Sleep. 36 (2): 203–220. doi:10.5665/sleep.2374. PMC 3543053. PMID 23372268.
- ^ a b Olaithe, Michelle; Bucks, Romola S.; Hillman, David R.; Eastwood, Peter R. (April 2018). "Cognitive deficits in obstructive sleep apnea: Insights from a meta-review and comparison with deficits observed in COPD, insomnia, and sleep deprivation" (PDF). Sleep Medicine Reviews. 38: 39–49. doi:10.1016/j.smrv.2017.03.005. PMID 28760549.
- ^ Rizzo D (August 2018). "Driving with obstructive sleep apnea policies, behaviors and screening measures".
{{cite journal}}: Cite journal requires|journal=(help) - ^ Morrone E, D'Artavilla Lupo N (2020). "Microsleep as a marker of sleepiness in obstructive sleep apnea patients". Journal of Sleep Research. 29 (2): e12882. doi:10.1111/jsr.12882. PMID 31180173. S2CID 182949124.
- ^ Cori JM, Jackson ML, Barnes M, Westlake J, Emerson P, Lee J, et al. (June 2018). "The Differential Effects of Regular Shift Work and Obstructive Sleep Apnea on Sleepiness, Mood and Neurocognitive Function". Journal of Clinical Sleep Medicine. 14 (6): 941–951. doi:10.5664/jcsm.7156. PMC 5991942. PMID 29852909.
- ^ Slater G, Steier J (December 2012). "Excessive daytime sleepiness in sleep disorders". Journal of Thoracic Disease. 4 (6): 608–616. doi:10.3978/j.issn.2072-1439.2012.10.07. PMC 3506799. PMID 23205286.
- ^ LaGrotte C, Fernandez-Mendoza J, Calhoun SL, Liao D, Bixler EO, Vgontzas AN (September 2016). "The relative association of obstructive sleep apnea, obesity and excessive daytime sleepiness with incident depression: a longitudinal, population-based study". International Journal of Obesity. 40 (9): 1397–1404. doi:10.1038/ijo.2016.87. PMC 5014694. PMID 27143032.
- ^ Mulgrew AT, Ryan CF, Fleetham JA, Cheema R, Fox N, Koehoorn M, et al. (December 2007). "The impact of obstructive sleep apnea and daytime sleepiness on work limitation". Sleep Medicine. 9 (1): 42–53. doi:10.1016/j.sleep.2007.01.009. PMID 17825611.
- ^ Guilleminault C, Brooks SN (August 2001). "Excessive daytime sleepiness: a challenge for the practising neurologist". Brain. 124 (Pt 8): 1482–1491. doi:10.1093/brain/124.8.1482. PMID 11459741.
- ^ Jackson ML, McEvoy RD, Banks S, Barnes M (January 2018). "Neurobehavioral Impairment and CPAP Treatment Response in Mild-Moderate Obstructive Sleep Apneas". Journal of Clinical Sleep Medicine. 14 (1): 47–56. doi:10.5664/jcsm.6878. PMC 5734892. PMID 29198304.
- ^ Eskandari D, Zou D, Grote L, Schneider H, Penzel T, Hedner J (June 2017). "Independent associations between arterial bicarbonate, apnea severity and hypertension in obstructive sleep apnea". Respiratory Research. 18 (1): 130. doi:10.1186/s12931-017-0607-9. PMC 5490198. PMID 28659192.
- ^ a b c Drager LF, Togeiro SM, Polotsky VY, Lorenzi-Filho G (August 2013). "Obstructive sleep apnea: a cardiometabolic risk in obesity and the metabolic syndrome". Journal of the American College of Cardiology. 62 (7): 569–76. doi:10.1016/j.jacc.2013.05.045. PMC 4461232. PMID 23770180.
- ^ a b Ashrafian H, Toma T, Rowland SP, Harling L, Tan A, Efthimiou E, et al. (July 2015). "Bariatric Surgery or Non-Surgical Weight Loss for Obstructive Sleep Apnoea? A Systematic Review and Comparison of Meta-analyses". Obesity Surgery. 25 (7): 1239–1250. doi:10.1007/s11695-014-1533-2. hdl:10044/1/54731. PMID 25537297. S2CID 5284156.
- ^ Dobrosielski DA, Patil S, Schwartz AR, Bandeen-Roche K, Stewart KJ (January 2015). "Effects of exercise and weight loss in older adults with obstructive sleep apnea". Medicine and Science in Sports and Exercise. 47 (1): 20–26. doi:10.1249/MSS.0000000000000387. PMC 4246024. PMID 24870569.
- ^ a b c d Reutrakul, Sirimon; Mokhlesi, Babak (November 2017). "Obstructive Sleep Apnea and Diabetes". Chest. 152 (5): 1070–1086. doi:10.1016/j.chest.2017.05.009. PMC 5812754. PMID 28527878.
- ^ a b c Guglielmi O, Magnavita N, Garbarino S (May 2018). "Sleep quality, obstructive sleep apnea, and psychological distress in truck drivers: a cross-sectional study". Social Psychiatry and Psychiatric Epidemiology. 53 (5): 531–536. doi:10.1007/s00127-017-1474-x. PMID 29285594. S2CID 4914966.
- ^ a b c Olaithe M, Nanthakumar S, Eastwood PR, Bucks RS (2015). "Cognitive and Mood Dysfunction in Adult Obstructive Sleep Apnea (OSA): Implications for Psychological Research and Practice". Translational Issues in Psychological Science. 1 (1): 67–78. doi:10.1037/tps0000021.
- ^ a b c Garbarino S, Bardwell WA, Guglielmi O, Chiorri C, Bonanni E, Magnavita N (2018). "Association of Anxiety and Depression in Obstructive Sleep Apnea Patients: A Systematic Review and Meta-Analysis". Behavioral Sleep Medicine. 18 (1): 35–57. doi:10.1080/15402002.2018.1545649. PMID 30453780. S2CID 53872339.
- ^ a b Xiao Y, Han D, Zang H, Wang D (June 2016). "The effectiveness of nasal surgery on psychological symptoms in patients with obstructive sleep apnea and nasal obstruction". Acta Oto-Laryngologica. 136 (6): 626–632. doi:10.3109/00016489.2016.1143120. PMID 26903174. S2CID 10364903.
- ^ Gupta MA, Simpson FC (January 2015). "Obstructive sleep apnea and psychiatric disorders: a systematic review". Journal of Clinical Sleep Medicine. 11 (2): 165–175. doi:10.5664/jcsm.4466. PMC 4298774. PMID 25406268.
- ^ Jonas DE, Amick HR, Feltner C, Weber RP, Arvanitis M, Stine A, et al. (January 2017). "Screening for Obstructive Sleep Apnea in Adults: Evidence Report and Systematic Review for the US Preventive Services Task Force". JAMA. 317 (4): 415–433. doi:10.1001/jama.2016.19635. PMID 28118460. S2CID 3656829.
- ^ a b Charokopos A, Card ME, Gunderson C, Steffens C, Bastian LA (September 2018). "The Association of Obstructive Sleep Apnea and Pain Outcomes in Adults: A Systematic Review". Pain Medicine. 19 (suppl_1): S69–S75. doi:10.1093/pm/pny140. PMID 30203008. S2CID 52182664.
- ^ Jonas DE, Amick HR, Feltner C, Weber RP, Arvanitis M, Stine A, et al. (January 2017). "Screening for Obstructive Sleep Apnea in Adults: Evidence Report and Systematic Review for the US Preventive Services Task Force". JAMA. 317 (4): 415–433. doi:10.1001/jama.2016.19635. PMID 28118460.
- ^ Bibbins-Domingo K, Grossman DC, Curry SJ, Davidson KW, Epling JW, García FA, et al. (January 2017). "Screening for Obstructive Sleep Apnea in Adults: US Preventive Services Task Force Recommendation Statement". JAMA. 317 (4): 407–414. doi:10.1001/jama.2016.20325. PMID 28118461.
- ^ a b Franklin KA, Lindberg E (August 2015). "Obstructive sleep apnea is a common disorder in the population-a review on the epidemiology of sleep apnea". Journal of Thoracic Disease. 7 (8): 1311–1322. doi:10.3978/j.issn.2072-1439.2015.06.11. PMC 4561280. PMID 26380759.
- ^ Berry RB, Quan SF, Abrue AR, et al.; for the American Academy of Sleep Medicine. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. Version 2.6. Darien, IL: American Academy of Sleep Medicine; 2020.
- ^ Kapur VK, Auckley DH, Chowdhuri S, Kuhlmann DC, Mehra R, Ramar K, Harrod CG (March 2017). "Clinical Practice Guideline for Diagnostic Testing for Adult Obstructive Sleep Apnea: An American Academy of Sleep Medicine Clinical Practice Guideline". Journal of Clinical Sleep Medicine. 13 (3): 479–504. doi:10.5664/jcsm.6506. PMC 5337595. PMID 28162150.
- ^ Thurnheer, R. (September 2007). "Diagnosis of the obstructive sleep apnea syndrome". Minerva Pneumologica. 46 (3): 191–204. S2CID 52540419.
- ^ Crook S, Sievi NA, Bloch KE, Stradling JR, Frei A, Puhan MA, Kohler M (April 2019). "Minimum important difference of the Epworth Sleepiness Scale in obstructive sleep apnoea: estimation from three randomised controlled trials". Thorax. 74 (4): 390–396. doi:10.1136/thoraxjnl-2018-211959. PMID 30100576. S2CID 51967356.
- ^ Chiu HY, Chen PY, Chuang LP, Chen NH, Tu YK, Hsieh YJ, et al. (December 2017). "Diagnostic accuracy of the Berlin questionnaire, STOP-BANG, STOP, and Epworth sleepiness scale in detecting obstructive sleep apnea: A bivariate meta-analysis". Sleep Medicine Reviews. 36: 57–70. doi:10.1016/j.smrv.2016.10.004. PMID 27919588.
- ^ Amra B, Javani M, Soltaninejad F, Penzel T, Fietze I, Schoebel C, Farajzadegan Z (2018). "Comparison of Berlin Questionnaire, STOP-Bang, and Epworth Sleepiness Scale for Diagnosing Obstructive Sleep Apnea in Persian Patients". International Journal of Preventive Medicine. 9 (28): 28. doi:10.4103/ijpvm.IJPVM_131_17. PMC 5869953. PMID 29619152.
- ^ American Academy of Sleep Medicine. International classification of sleep disorders. 3rd ed. Darien, IL: American Academy of Sleep Medicine, 2014.[page needed]
- ^ Tschopp, Samuel; Wimmer, Wilhelm; Caversaccio, Marco; Borner, Urs; Tschopp, Kurt (2021-03-30). "Night-to-night variability in obstructive sleep apnea using peripheral arterial tonometry: a case for multiple night testing". Journal of Clinical Sleep Medicine. 17 (9): 1751–1758. doi:10.5664/jcsm.9300. ISSN 1550-9389. PMC 8636340. PMID 33783347. S2CID 232420123.
- ^ Jennifer M. Slowik; Jacob F. Collen (2020). "Obstructive Sleep Apnea". StatPearls. PMID 29083619.
 This article incorporates text available under the CC BY 4.0 license.
This article incorporates text available under the CC BY 4.0 license.
- ^ "Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The Report of an American Academy of Sleep Medicine Task Force". Sleep. 22 (5): 667–689. August 1999. doi:10.1093/sleep/22.5.667. PMID 10450601.
- ^ Mulgrew AT, Fox N, Ayas NT, Ryan CF (February 2007). "Diagnosis and initial management of obstructive sleep apnea without polysomnography: a randomized validation study". Annals of Internal Medicine. 146 (3): 157–166. doi:10.7326/0003-4819-146-3-200702060-00004. PMID 17283346. S2CID 25693423.
- ^ Flemons WW, Whitelaw WA, Brant R, Remmers JE (November 1994). "Likelihood ratios for a sleep apnea clinical prediction rule". American Journal of Respiratory and Critical Care Medicine. 150 (5 Pt 1): 1279–1285. doi:10.1164/ajrccm.150.5.7952553. PMID 7952553.
- ^ a b Friedman: Sleep Apnea and Snoring, 1st ed. 2008
- ^ Azagra-Calero E, Espinar-Escalona E, Barrera-Mora JM, Llamas-Carreras JM, Solano-Reina E (November 2012). "Obstructive sleep apnea syndrome (OSAS). Review of the literature". Medicina Oral, Patologia Oral y Cirugia Bucal. 17 (6): e925–929. doi:10.4317/medoral.17706. PMC 3505711. PMID 22549673.
- ^ a b c d e f Qaseem, Amir; Holty, Jon-Erik C.; Owens, Douglas K.; Dallas, Paul; Starkey, Melissa; Shekelle, Paul; Clinical Guidelines Committee of the American College of, Physicians. (1 October 2013). "Management of Obstructive Sleep Apnea in Adults: A Clinical Practice Guideline From the American College of Physicians". Annals of Internal Medicine. 159 (7): 471–483. doi:10.7326/0003-4819-159-7-201310010-00704. PMID 24061345. S2CID 6241714.
- ^ Bratton DJ, Gaisl T, Wons AM, Kohler M (December 2015). "CPAP vs Mandibular Advancement Devices and Blood Pressure in Patients With Obstructive Sleep Apnea: A Systematic Review and Meta-analysis". JAMA. 314 (21): 2280–2293. doi:10.1001/jama.2015.16303. PMID 26624827.
- ^ Iftikhar IH, Kline CE, Youngstedt SD (February 2014). "Effects of exercise training on sleep apnea: a meta-analysis". Lung. 192 (1): 175–184. doi:10.1007/s00408-013-9511-3. PMC 4216726. PMID 24077936.
- ^ Browaldh N, Nerfeldt P, Lysdahl M, Bring J, Friberg D (September 2013). "SKUP3 randomised controlled trial: polysomnographic results after uvulopalatopharyngoplasty in selected patients with obstructive sleep apnoea". Thorax. 68 (9): 846–853. doi:10.1136/thoraxjnl-2012-202610. PMID 23644225. S2CID 27682387.
- ^ a b Stuck, Boris A.; Ravesloot, Madeline J. L.; Eschenhagen, Till; de Vet, H. C. W.; Sommer, J. Ulrich (1 October 2018). "Uvulopalatopharyngoplasty with or without tonsillectomy in the treatment of adult obstructive sleep apnea – A systematic review". Sleep Medicine. 50: 152–165. doi:10.1016/j.sleep.2018.05.004. PMID 30056286. S2CID 51878426.
- ^ a b Giles TL, Lasserson TJ, Smith BH, White J, Wright J, Cates CJ (July 2006). Giles T (ed.). "Continuous positive airways pressure for obstructive sleep apnoea in adults". The Cochrane Database of Systematic Reviews (3): CD001106. doi:10.1002/14651858.CD001106.pub3. PMID 16855960.
- ^ a b c d Askland, Kathleen; Wright, Lauren; Wozniak, Dariusz R; Emmanuel, Talia; Caston, Jessica; Smith, Ian (7 April 2020). "Educational, supportive and behavioural interventions to improve usage of continuous positive airway pressure machines in adults with obstructive sleep apnoea". Cochrane Database of Systematic Reviews. 2020 (4): CD007736. doi:10.1002/14651858.CD007736.pub3. PMC 7137251. PMID 32255210.
- ^ Riaz M, Certal V, Nigam G, Abdullatif J, Zaghi S, Kushida CA, Camacho M (2015). "Nasal Expiratory Positive Airway Pressure Devices (Provent) for OSA: A Systematic Review and Meta-Analysis". Sleep Disorders. 2015: 734798. doi:10.1155/2015/734798. PMC 4699057. PMID 26798519.
- ^ Whitelaw, William A.; Brant, Rollin F.; Flemons, W. Ward (15 January 2005). "Clinical Usefulness of Home Oximetry Compared with Polysomnography for Assessment of Sleep Apnea". American Journal of Respiratory and Critical Care Medicine. 171 (2): 188–193. doi:10.1164/rccm.200310-1360OC. PMID 15486338. Review in: Caples SM (2005). "The accuracy of physicians in predicting successful treatment response in suspected obstructive sleep apnea did not differ between home monitoring and polysomnography". ACP Journal Club. 143 (1): 21. doi:10.7326/ACPJC-2005-143-1-021. PMID 15989309. S2CID 219673066.
- ^ Littner M, Hirshkowitz M, Davila D, Anderson WM, Kushida CA, Woodson BT, et al. (March 2002). "Practice parameters for the use of auto-titrating continuous positive airway pressure devices for titrating pressures and treating adult patients with obstructive sleep apnea syndrome. An American Academy of Sleep Medicine report". Sleep. 25 (2): 143–147. doi:10.1093/sleep/25.2.143. PMID 11902424.
- ^ McNicholas WT, Bonsignore MR, Lévy P, Ryan S (October 2016). "Mild obstructive sleep apnoea: clinical relevance and approaches to management". The Lancet. Respiratory Medicine. 4 (10): 826–834. doi:10.1016/S2213-2600(16)30146-1. PMID 27245915.
- ^ Neill AM, Angus SM, Sajkov D, McEvoy RD (January 1997). "Effects of sleep posture on upper airway stability in patients with obstructive sleep apnea". American Journal of Respiratory and Critical Care Medicine. 155 (1): 199–204. doi:10.1164/ajrccm.155.1.9001312. PMID 9001312.
- ^ Nakano H, Ikeda T, Hayashi M, Ohshima E, Onizuka A (March 2003). "Effects of body position on snoring in apneic and nonapneic snorers". Sleep. 26 (2): 169–172. doi:10.1093/sleep/26.2.169. PMID 12683476.
- ^ Loord H, Hultcrantz E (August 2007). "Positioner--a method for preventing sleep apnea". Acta Oto-Laryngologica. 127 (8): 861–868. doi:10.1080/00016480601089390. PMID 17762999. S2CID 323418.
- ^ Szollosi I, Roebuck T, Thompson B, Naughton MT (August 2006). "Lateral sleeping position reduces severity of central sleep apnea / Cheyne-Stokes respiration". Sleep. 29 (8): 1045–1051. doi:10.1093/sleep/29.8.1045. PMID 16944673.
- ^ Caba, Justin (16 April 2015). "How The Saxophone Could Solve Your Sleep Apnea". Medical Daily.
- ^ Ward, Christopher P.; York, Kaki M.; McCoy, John G. (15 June 2012). "Risk of Obstructive Sleep Apnea Lower in Double Reed Wind Musicians". Journal of Clinical Sleep Medicine. 08 (3): 251–255. doi:10.5664/jcsm.1906. PMC 3365082. PMID 22701381.
- ^ Li, Qiming; Tang, Hongyi; Liu, Xueye; Luo, Qing; Jiang, Zhe; Martin, Domingo; Guo, Jing (1 May 2020). "Comparison of dimensions and volume of upper airway before and after mini-implant assisted rapid maxillary expansion". The Angle Orthodontist. 90 (3): 432–441. doi:10.2319/080919-522.1. PMC 8032299. PMID 32045299.
- ^ Abdullatif, Jose; Certal, Victor; Zaghi, Soroush; Song, Sungjin A.; Chang, Edward T.; Gillespie, M. Boyd; Camacho, Macario (1 May 2016). "Maxillary expansion and maxillomandibular expansion for adult OSA: A systematic review and meta-analysis". Journal of Cranio-Maxillofacial Surgery. 44 (5): 574–578. doi:10.1016/j.jcms.2016.02.001. PMID 26948172.
- ^ Browaldh, Nanna; Nerfeldt, Pia; Lysdahl, Michael; Bring, Johan; Friberg, Danielle (1 September 2013). "SKUP3 randomised controlled trial: polysomnographic results after uvulopalatopharyngoplasty in selected patients with obstructive sleep apnoea". Thorax. 68 (9): 846–853. doi:10.1136/thoraxjnl-2012-202610. PMID 23644225. S2CID 27682387.
- ^ a b Tschopp, Samuel; Tschopp, Kurt (2019). "Tonsil size and outcome of uvulopalatopharyngoplasty with tonsillectomy in obstructive sleep apnea". The Laryngoscope. 129 (12): E449–E454. doi:10.1002/lary.27899. ISSN 1531-4995. PMID 30848478. S2CID 73503702.
- ^ "Sleep apnea". University of Maryland Medical Center. Archived from the original on 2008-04-30.
- ^ Borgström, Anna; Nerfeldt, Pia; Friberg, Danielle (1 April 2017). "Adenotonsillotomy Versus Adenotonsillectomy in Pediatric Obstructive Sleep Apnea: An RCT". Pediatrics. 139 (4): e20163314. doi:10.1542/peds.2016-3314. PMID 28320866. S2CID 23443654.
- ^ "Premarket Approval (PMA)". Archived from the original on 2014-05-06. Retrieved 2014-05-05.. FDA "Premarket Approval (PMA) Inspire II Upper Airway Stimulation System" U.S. Food and Drug Administration. April 30, 2014.
- ^ Strollo, Patrick J.; Soose, Ryan J.; Maurer, Joachim T.; de Vries, Nico; Cornelius, Jason; Froymovich, Oleg; Hanson, Ronald D.; Padhya, Tapan A.; Steward, David L.; Gillespie, M. Boyd; Woodson, B. Tucker; Van de Heyning, Paul H.; Goetting, Mark G.; Vanderveken, Olivier M.; Feldman, Neil; Knaack, Lennart; Strohl, Kingman P. (9 January 2014). "Upper-Airway Stimulation for Obstructive Sleep Apnea". New England Journal of Medicine. 370 (2): 139–149. doi:10.1056/NEJMoa1308659. PMID 24401051. S2CID 13764858.
- ^ a b Farrar J, Ryan J, Oliver E, Gillespie MB (October 2008). "Radiofrequency ablation for the treatment of obstructive sleep apnea: a meta-analysis". The Laryngoscope. 118 (10): 1878–1883. doi:10.1097/MLG.0b013e31817d9cc1. PMID 18806478. S2CID 9844227.
- ^ Eick OJ (July 2002). "Temperature controlled radiofrequency ablation". Indian Pacing and Electrophysiology Journal. 3. 2 (3): 66–73. PMC 1564057. PMID 17006561.
- ^ Bashetty K, Nadig G, Kapoor S (October 2009). "Electrosurgery in aesthetic and restorative dentistry: A literature review and case reports". Journal of Conservative Dentistry. 12 (4): 139–144. doi:10.4103/0972-0707.58332. PMC 2879725. PMID 20543922.
- ^ "Position Statement: Submucosal Ablation of the Tongue Base for OSAS". American Academy of Otolaryngology-Head and Neck Surgery. 20 March 2014.
- ^ Weaver, T. E.; Grunstein, R. R. (15 February 2008). "Adherence to Continuous Positive Airway Pressure Therapy: The Challenge to Effective Treatment". Proceedings of the American Thoracic Society. 5 (2): 173–178. doi:10.1513/pats.200708-119MG. PMC 2645251. PMID 18250209.
- ^ Steward, David L.; Weaver, Edward M.; Woodson, B. Tucker (17 May 2016). "Multilevel Temperature-Controlled Radiofrequency for Obstructive Sleep Apnea: Extended Follow-Up". Otolaryngology–Head and Neck Surgery. 132 (4): 630–635. doi:10.1016/j.otohns.2004.11.013. PMID 15806059. S2CID 17909484.
- ^ Gaisl, Thomas; Haile, Sarah R.; Thiel, Sira; Osswald, Martin; Kohler, Malcolm (August 2019). "Efficacy of pharmacotherapy for OSA in adults: A systematic review and network meta-analysis". Sleep Medicine Reviews. 46: 74–86. doi:10.1016/j.smrv.2019.04.009. PMID 31075665. S2CID 149455430.
- ^ Gottlieb, Daniel J.; Punjabi, Naresh M. (14 April 2020). "Diagnosis and Management of Obstructive Sleep Apnea: A Review". JAMA. 323 (14): 1389–1400. doi:10.1001/jama.2020.3514. PMID 32286648. S2CID 215759986.
- ^ Veasey, Sigrid C. (1 February 2003). "Serotonin Agonists and Antagonists in Obstructive Sleep Apnea". American Journal of Respiratory Medicine. 2 (1): 21–29. doi:10.1007/BF03256636. PMID 14720019. S2CID 8288291.
- ^ Babson, Kimberly A.; Sottile, James; Morabito, Danielle (27 March 2017). "Cannabis, Cannabinoids, and Sleep: a Review of the Literature". Current Psychiatry Reports. 19 (4): 23. doi:10.1007/s11920-017-0775-9. PMID 28349316. S2CID 7429763.
- ^ Carley, David W.; Prasad, Bharati; Reid, Kathryn J.; Malkani, Roneil; Attarian, Hryar; Abbott, Sabra M.; Vern, Boris; Xie, Hui; Yuan, Chengbo; Zee, Phyllis C. (1 January 2018). "Pharmacotherapy of Apnea by Cannabimimetic Enhancement, the PACE Clinical Trial: Effects of Dronabinol in Obstructive Sleep Apnea". Sleep. 41 (1). doi:10.1093/sleep/zsx184. PMC 5806568. PMID 29121334.
- ^ a b Ramar, Kannan; Rosen, Ilene M.; Kirsch, Douglas B.; Chervin, Ronald D.; Carden, Kelly A.; Aurora, R. Nisha; Kristo, David A.; Malhotra, Raman K.; Martin, Jennifer L.; Olson, Eric J.; Rosen, Carol L.; Rowley, James A. (15 April 2018). "Medical Cannabis and the Treatment of Obstructive Sleep Apnea: An American Academy of Sleep Medicine Position Statement". Journal of Clinical Sleep Medicine. 14 (4): 679–681. doi:10.5664/jcsm.7070. PMC 5886446. PMID 29609727.
- ^ Kolla, Bhanu Prakash; Mansukhani, Meghna P.; Olson, Eric J.; St. Louis, Erik K.; Silber, Michael H.; Morgenthaler, Timothy I. (June 2018). "Medical Cannabis for Obstructive Sleep Apnea: Premature and Potentially Harmful". Mayo Clinic Proceedings. 93 (6): 689–692. doi:10.1016/j.mayocp.2018.04.011. PMID 29866280. S2CID 46936292.
- ^ Ramar, Kannan; Kirsch, Douglas B.; Carden, Kelly A.; Rosen, Ilene M.; Malhotra, Raman K. (15 October 2018). "Medical Cannabis, Synthetic Marijuana Extracts, and Obstructive Sleep Apnea". Journal of Clinical Sleep Medicine. 14 (10): 1815–1816. doi:10.5664/jcsm.7412. PMC 6175803. PMID 30353829.
- ^ Malhotra, Atul; Grunstein, Ronald R.; Fietze, Ingo; Weaver, Terri E.; Redline, Susan; Azarbarzin, Ali; Sands, Scott A.; Schwab, Richard J.; Dunn, Julia P.; Chakladar, Sujatro; Bunck, Mathijs C.; Bednarik, Josef (2024-06-21). "Tirzepatide for the Treatment of Obstructive Sleep Apnea and Obesity". New England Journal of Medicine. doi:10.1056/NEJMoa2404881. ISSN 0028-4793.
- ^ "Obstructive Sleep Apnea (OSA) Medication: CNS stimulants, Dopamine/Norepinephrine Reuptake Inhibitors". emedicine.medscape.com. Retrieved 2024-08-15.
- ^ Greenblatt, Karl; Adams, Ninos (2024), "Modafinil", StatPearls, Treasure Island (FL): StatPearls Publishing, PMID 30285371, retrieved 2024-08-15
- ^ "Sunosi: Side effects, alternatives, uses, dosage, and more". www.medicalnewstoday.com. 2020-09-23. Retrieved 2024-08-15.
- ^ Carvalho, Fernando R; Lentini-Oliveira, Débora A; Prado, Lucila BF; Prado, Gilmar F; Carvalho, Luciane BC (4 October 2016). "Oral appliances and functional orthopaedic appliances for obstructive sleep apnoea in children". Cochrane Database of Systematic Reviews. 10 (11): CD005520. doi:10.1002/14651858.CD005520.pub3. PMC 6458031. PMID 27701747.
- ^ Shah NA, Botros NA, Yaggi HK, Mohsenin V (May 20, 2007). "Sleep Apnea Increases Risk of Heart Attack or Death by 30%" (Press release). New Haven, Connecticut: American Thoracic Society. Archived from the original on May 24, 2009.
- ^ Sun, Yi; Yuan, Hai; Zhao, Miao-Qing; Wang, Yan; Xia, Ming; Li, Yan-Zhong (April 2014). "Cardiac structural and functional changes in old elderly patients with obstructive sleep apnoea–hypopnoea syndrome". Journal of International Medical Research. 42 (2): 395–404. doi:10.1177/0300060513502890. PMID 24445697. S2CID 23011075.
- ^ Silverberg DS, Iaina A, Oksenberg A (January 2002). "Treating obstructive sleep apnea improves essential hypertension and quality of life". American Family Physician. 65 (2): 229–236. PMID 11820487.
- ^ Grigg-Damberger, Madeleine (February 2006). "Why a Polysomnogram Should Become Part of the Diagnostic Evaluation of Stroke and Transient Ischemic Attack". Journal of Clinical Neurophysiology. 23 (1): 21–38. doi:10.1097/01.wnp.0000201077.44102.80. PMID 16514349. S2CID 19626174.
- ^ Gaisl T, Bratton DJ, Kohler M (August 2015). "The impact of obstructive sleep apnoea on the aorta". The European Respiratory Journal. 46 (2): 532–544. doi:10.1183/09031936.00029315. PMID 26113685. S2CID 14458813.
- ^ Peppard, Paul E.; Young, Terry; Palta, Mari; Skatrud, James (11 May 2000). "Prospective Study of the Association between Sleep-Disordered Breathing and Hypertension". New England Journal of Medicine. 342 (19): 1378–1384. doi:10.1056/NEJM200005113421901. PMID 10805822.
- ^ Lavie, P.; Herer, P; Hoffstein, V (19 February 2000). "Obstructive sleep apnoea syndrome as a risk factor for hypertension: population study". BMJ. 320 (7233): 479–482. doi:10.1136/bmj.320.7233.479. PMC 27290. PMID 10678860.
- ^ Yaggi, H. Klar; Concato, John; Kernan, Walter N.; Lichtman, Judith H.; Brass, Lawrence M.; Mohsenin, Vahid (10 November 2005). "Obstructive Sleep Apnea as a Risk Factor for Stroke and Death". New England Journal of Medicine. 353 (19): 2034–2041. doi:10.1056/NEJMoa043104. PMID 16282178. S2CID 23360654.
- ^ Schröder, Carmen M; O'Hara, Ruth (2005). "Depression and Obstructive Sleep Apnea (OSA)". Annals of General Psychiatry. 4 (1): 13. doi:10.1186/1744-859X-4-13. PMC 1181621. PMID 15982424.
- ^ Gagnon K, Baril AA, Gagnon JF, Fortin M, Décary A, Lafond C, et al. (October 2014). "Cognitive impairment in obstructive sleep apnea". Pathologie-Biologie. 62 (5): 233–240. doi:10.1016/j.patbio.2014.05.015. PMID 25070768.
- ^ Lal C, Strange C, Bachman D (June 2012). "Neurocognitive impairment in obstructive sleep apnea". Chest. 141 (6): 1601–1610. doi:10.1378/chest.11-2214. PMID 22670023.
- ^ a b Punjabi NM (February 2008). "The epidemiology of adult obstructive sleep apnea". Proceedings of the American Thoracic Society. 5 (2): 136–43. doi:10.1513/pats.200709-155MG. PMC 2645248. PMID 18250205.
- ^ a b c d Senaratna CV, Perret JL, Lodge CJ, Lowe AJ, Campbell BE, Matheson MC, et al. (August 2017). "Prevalence of obstructive sleep apnea in the general population: A systematic review". Sleep Medicine Reviews. 34: 70–81. doi:10.1016/j.smrv.2016.07.002. PMID 27568340.
- ^ Shamsuzzaman AS, Gersh BJ, Somers VK (October 2003). "Obstructive sleep apnea: implications for cardiac and vascular disease". JAMA. 290 (14): 1906–14. doi:10.1001/jama.290.14.1906. PMID 14532320.
- ^ Kezirian EJ, Boudewyns A, Eisele DW, Schwartz AR, Smith PL, Van de Heyning PH, De Backer WA (October 2010). "Electrical stimulation of the hypoglossal nerve in the treatment of obstructive sleep apnea". Sleep Medicine Reviews. 14 (5): 299–305. doi:10.1016/j.smrv.2009.10.009. PMID 20116305.
- ^ "ImThera aura6000 System for Sleep Apnea Gets a Go in Europe". Medgadget. 15 March 2012.
- ^ Puhan, Milo A.; Suarez, Alex; Cascio, Christian Lo; Zahn, Alfred; Heitz, Markus; Braendli, Otto (2 February 2006). "Didgeridoo playing as alternative treatment for obstructive sleep apnoea syndrome: randomised controlled trial". BMJ. 332 (7536): 266–270. doi:10.1136/bmj.38705.470590.55. PMC 1360393. PMID 16377643.
- ^ Guimarães, Kátia C.; Drager, Luciano F.; Genta, Pedro R.; Marcondes, Bianca F.; Lorenzi-Filho, Geraldo (15 May 2009). "Effects of Oropharyngeal Exercises on Patients with Moderate Obstructive Sleep Apnea Syndrome". American Journal of Respiratory and Critical Care Medicine. 179 (10): 962–966. doi:10.1164/rccm.200806-981OC. PMID 19234106.
