Benzocyclobutene

| |
| |
| Names | |
|---|---|
| Preferred IUPAC name
Bicyclo[4.2.0]octa-1,3,5-triene | |
| Other names
Benzocyclobutane
BCB Benzocyclobutene (not in accordance with IUPAC nomenclature) | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.161.355 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C8H8 | |
| Molar mass | 104.152 g·mol−1 |
| Density | 0.957 g/cm3 |
| Boiling point | 150 °C (302 °F; 423 K) |
Refractive index (nD)
|
1.541 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Benzocyclobutene (BCB) is a benzene ring fused to a cyclobutane ring. It has chemical formula C8H8.[1]
BCB is frequently used to create photosensitive polymers. BCB-based polymer dielectrics may be spun on or applied to various substrates for use in Micro Electro-Mechanical Systems (MEMS) and microelectronics processing. Applications include wafer bonding, optical interconnects, low-κ dielectrics, or even intracortical neural implants.
Reactions
[edit]Benzocyclobutene is a strained system which, upon heating to approximately 180 °C, causes the cyclobutene to undergo a conrotatory ring-opening reaction, forming o-xylylene. Since this process destroys the aromaticity of the benzene ring, the reverse reaction is highly favored.
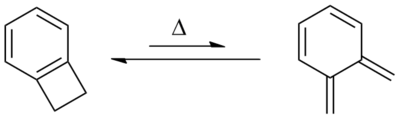
o-Xylylenes generated in this way have been used prolifically in cycloaddition reactions, which restore the aromaticity to the benzene ring, while forming a new annulated species.[2]
Uses
[edit]The benzocyclobutene moiety has also appeared in a number of chemical compounds with pharmacological properties such as ivabradine and S33005. Additionally, the benzocyclobutene analog of 2C-B has been prepared[3] and a benzocyclobutene-derived amphetamine has been patented.[4]
See also
[edit]References
[edit]- ^ 164410 Benzocyclobutene 98%
- ^ Mehta, G.; Kotha, S. (2001). "Recent chemistry of benzocyclobutenes" (PDF). Tetrahedron Lett. 57 (4): 625–659. doi:10.1016/s0040-4020(00)00958-3.
- ^ "The Binding Database".
- ^ US 3149159, "Substituted 7-aminoalkylbicyclo-[4. 2. 0]octa-1,3,5-trienes"
